Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches
- PMID: 24384237
- PMCID: PMC3905086
- DOI: 10.5732/cjc.013.10217
Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches
Abstract
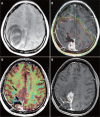
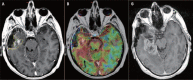
The current standards in radiotherapy of high-grade gliomas (HGG) are based on anatomic imaging techniques, usually computed tomography (CT) scanning and magnetic resonance imaging (MRI). The guidelines vary depending on whether the HGG is a histological grade 3 anaplastic glioma (AG) or a grade 4 glioblastoma multiforme (GBM). For AG, T2-weighted MRI sequences plus the region of contrast enhancement in T1 are considered for the delineation of the gross tumor volume (GTV), and an isotropic expansion of 15 to 20 mm is recommended for the clinical target volume (CTV). For GBM, the Radiation Therapy Oncology Group favors a two-step technique, with an initial phase (CTV1) including any T2 hyperintensity area (edema) plus a 20 mm margin treated with up to 46 Gy in 23 fractions, followed by a reduction in CTV2 to the contrast enhancement region in T1 with an additional 25 mm margin. The European Organisation of Research and Treatment of Cancer recommends a single-phase technique with a unique GTV, which comprises the T1 contrast enhancement region plus a margin of 20 to 30 mm. A total dose of 60 Gy in 30 fractions is usually delivered for GBM, and a dose of 59.4 Gy in 33 fractions is typically given for AG. As more than 85% of HGGs recur in field, dose-escalation studies have shown that 70 to 75 Gy can be delivered in 6 weeks with relevant toxicities developing in <10% of the patients. However, the only randomized dose-escalation trial, in which the boost dose was guided by conventional MRI, did not show any survival advantage of this treatment over the reference arm. HGGs are amongst the most infiltrative and heterogeneous tumors, and it was hypothesized that the most highly aggressive areas were missed; thus, better visualization of these high-risk regions for radiation boost could decrease the recurrence rate. Innovations in imaging and linear accelerators (LINAC) could help deliver the right doses of radiation to the right subvolumes according to the dose-painting concept. Advanced imaging techniques provide functional information on cellular density (diffusion MRI), angiogenesis (perfusion MRI), metabolic activity and cellular proliferation [positron emission tomography (PET) and magnetic resonance spectroscopy (MRS)]. All of these non-invasive techniques demonstrated good association between the images and histology, with up to 40% of HGGs functionally presenting a high activity within the non-contrast-enhanced areas in T1. New LINAC technologies, such as intensity-modulated and stereotactic radiotherapy, help to deliver a simultaneous integrated boost (SIB) > 60 Gy. Trials delivering a SIB into a biological GTV showed the feasibility of this treatment, but the final results, in terms of clinical benefits for HGG patients, are still pending. Many issues have been identified: the variety of MRI and PET machines (and amino-acid tracers), the heterogeneity of the protocols used for image acquisition and post-treatment, the geometric distortion and the unreliable algorithms for co-registration of brain anatomy with functional maps, and the semi-quiescent but highly invasive HGG cells. These issues could be solved by the homogenization of the protocols and software applications, the simultaneous acquisition of anatomic and functional images (PET-MRI machines), the combination of complementary imaging tools (perfusion and diffusion MRI), and the concomitant addition of some ad hoc targeted drugs against angiogenesis and invasiveness to chemoradiotherapy. The integration of these hybrid data will construct new synthetic metrics for fully individualized treatments.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
