Oral-aboral axis specification in the sea urchin embryo, IV: hypoxia radializes embryos by preventing the initial spatialization of nodal activity
- PMID: 24384388
- PMCID: PMC3929957
- DOI: 10.1016/j.ydbio.2013.12.035
Oral-aboral axis specification in the sea urchin embryo, IV: hypoxia radializes embryos by preventing the initial spatialization of nodal activity
Abstract
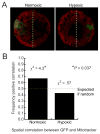
The oral-aboral axis of the sea urchin embryo is specified conditionally via a regulated feedback circuit involving the signaling gene nodal and its antagonist lefty. In normal development nodal activity becomes localized to the prospective oral side of the blastula stage embryo, a process that requires lefty. In embryos of Strongylocentrotus purpuratus, a redox gradient established by asymmetrically distributed mitochondria provides an initial spatial input that positions the localized domain of nodal expression. This expression is perturbed by hypoxia, leading to development of radialized embryos lacking an oral-aboral axis. Here we show that this radialization is not caused by a failure to express nodal, but rather by a failure to localize nodal activity to one side of the embryo. This occurs even when embryos are removed from hypoxia at late cleavage stage when nodal is first expressed, indicating that the effect involves the initiation phase of nodal activity, rather than its positive feedback-driven amplification and maintenance. Quantitative fluorescence microscopy of MitoTracker Orange-labeled embryos expressing nodal-GFP reporter gene revealed that hypoxia abolishes the spatial correlation between mitochondrial distribution and nodal expression, suggesting that hypoxia eliminates the initial spatial bias in nodal activity normally established by the redox gradient. We propose that absent this bias, the initiation phase of nodal expression is spatially uniform, such that the ensuing Nodal-mediated community effect is not localized, and hence refractory to Lefty-mediated enforcement of localization.
Keywords: Axis; Embryo; Hypoxia; Mitochondria; Nodal; Polarity.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

