Ubiquitin-dependent sorting in endocytosis
- PMID: 24384571
- PMCID: PMC3941215
- DOI: 10.1101/cshperspect.a016808
Ubiquitin-dependent sorting in endocytosis
Abstract
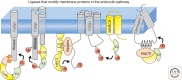
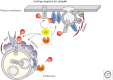
When ubiquitin (Ub) is attached to membrane proteins on the plasma membrane, it directs them through a series of sorting steps that culminate in their delivery to the lumen of the lysosome where they undergo complete proteolysis. Ubiquitin is recognized by a series of complexes that operate at a number of vesicle transport steps. Ubiquitin serves as a sorting signal for internalization at the plasma membrane and is the major signal for incorporation into intraluminal vesicles of multivesicular late endosomes. The sorting machineries that catalyze these steps can bind Ub via a variety of Ub-binding domains. At the same time, many of these complexes are themselves ubiquitinated, thus providing a plethora of potential mechanisms to regulate their activity. Here we provide an overview of how membrane proteins are selected for ubiquitination and deubiquitination within the endocytic pathway and how that ubiquitin signal is interpreted by endocytic sorting machineries.
Figures

References
-
- Ali N, Zhang L, Taylor S, Mironov A, Urbe S, Woodman P 2013. Recruitment of UBPY and ESCRT exchange drive HD-PTP-dependent sorting of EGFR to the MVB. Curr Biol 23: 453–461 - PubMed
-
- Alwan HA, van Leeuwen JE 2007. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J Biol Chem 282: 1658–1669 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
