Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis
- PMID: 24384681
- PMCID: PMC3915140
- DOI: 10.1038/bjc.2013.793
Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis
Abstract
Background: Circulating concentrations of the cytokines interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF) and chemokines monocyte chemotatic protein 1 (MCP-1)/CCL2 and growth-regulator oncogene α (GROα)/chemokine C-X-C motif ligand 1 are commonly increased in cancer patients and they are increasingly recognised as important promoters, via divergent mechanisms, of cancer progression and metastasis.
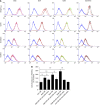
Methods: The effect of galectins-2, -4 and -8, whose circulating levels are highly increased in cancer patients, on endothelial secretion of cytokines was assessed in vitro by cytokine array and in mice. The relationship between serum levels of galectins and cytokines was analysed in colon and breast cancer patients.
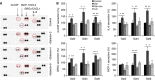
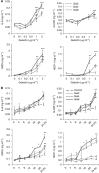
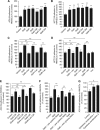
Results: Galectins-2, -4 and -8 at pathological concentrations induce secretion of G-CSF, IL-6, MCP-1 and GROα from the blood vascular endothelial cells in vitro and in mice. Multiple regression analysis indicates that increased circulation of these galectins accounts for 41∼83% of the variance of these cytokines in the sera of colon and breast cancer patients. The galectin-induced secretion of these cytokines/chemokines is shown to enhance the expression of endothelial cell surface adhesion molecules, causing increased cancer-endothelial adhesion and increased endothelial tubule formation.
Conclusion: The increased circulation of galectins -2, -4 and -8 in cancer patients contributes substantially to the increased circulation of G-CSF, IL-6 and MCP-1 by interaction with the blood vascular endothelium. These cytokines and chemokines in turn enhance endothelial cell activities in angiogenesis and metastasis.
Figures
References
-
- Bandapalli OR, Ehrmann F, Ehemann V, Gaida M, Macher-Goeppinger S, Wente M, Schirmacher P, Brand K. Down-regulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine. 2012;57 (1:46–53. - PubMed
-
- Barrow H, Guo X, Wandall HH, Pedersen JW, Fu B, Zhao Q, Chen C, Rhodes JM, Yu LG. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011;17 (22:7035–7046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

