Results of a 14-Week, Multicenter, Prospective, Randomized, Open-Label, Noninferiority Clinical Trial Comparing the Antihypertensive Effect and Edema Incidence of Lacidipine and Amlodipine in Older Korean Patients with Mild-to-Moderate Hypertension
- PMID: 24384734
- PMCID: PMC3862199
- DOI: 10.1016/j.curtheres.2013.02.001
Results of a 14-Week, Multicenter, Prospective, Randomized, Open-Label, Noninferiority Clinical Trial Comparing the Antihypertensive Effect and Edema Incidence of Lacidipine and Amlodipine in Older Korean Patients with Mild-to-Moderate Hypertension
Abstract
Background: It has been shown that administration of lacidipine markedly reduces systolic blood pressure in elderly patients with hypertension without increasing the incidence of cardiovascular events and total mortality. But in Korea, there were no available data about the effectiveness and safety of lacidipine.
Objectives: The goal of our study was to compare the effect of lacidipine and amlodipine besylate on sitting systolic blood pressure (SBP) and edema regression time as primary parameters, and sitting diastolic blood pressure (DBP) and tolerability as a secondary parameter in patients with hypertension.
Method: This was a prospective, randomized, open-label, noninferiority study in which patients received 14 weeks of treatment with either lacidipine or amlodipine besylate. Patients aged 55 to 80 years having uncomplicated, mild-to-moderate essential hypertension (SBP 140 to <180 mm Hg or DBP ≥90 mm Hg) and receiving no antihypertensive medications during the 2 weeks before randomization were randomly assigned to receive lacidipine or amlodipine. The incidence of adverse events was also assessed.
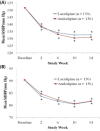
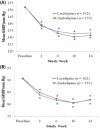
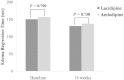
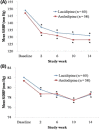
Results: In total, 315 patients (154 men, mean age 67.6 years) were included in the intent-to-treat analysis and randomly assigned to receive lacidipine (n = 162) or amlodipine besylate (n = 153); 286 patients were included in the per-protocol analysis (n = 150 for lacidipine, n = 136 for amlodipine) (12 in the lacidipine group and 17 in the amlodipine group were excluded from the per-protocol analysis due to consent withdrawal or protocol violation). There were no differences in demographic profiles between the 2 groups. Mean (SD) SBP changes at 14 weeks were -18.9 (12.7) mm Hg in the lacidipine group and -20.6 (12.4) mm Hg in the amlodipine group (P >0.05). Because the 1-sided 95% CI for the difference in mean SBP changes between groups (-4.18 to 0.72) was within the pre-specified lower limit (-5 mm Hg), lacidipine was considered noninferior to amlodipine. There were no differences in mean edema regression time and in mean DBP changes. These results were consistent in the isolated systolic hypertension subgroup analysis. The overall incidence of clinical adverse events was comparable between the 2 groups (ie, 7.4% in the lacidipine group and 11.1% in the amlodipine group [P >0.05]). The most common adverse events were headache and facial flushing (5 out of 162 patients [3.1%] in the lacidipine group and 11 out of 153 patients [7.2%] in the amlodipine group].
Conclusions: Fourteen weeks of lacidipine treatment significantly reduced blood pressure in older Korean patients with mild-to-moderate hypertension. The efficacy of lacidipine was not inferior to that of amlodipine besylate and tolerability was comparable between the 2 treatment groups. ClinicalTrials.gov identifier: NCT00460915.
Keywords: amlodipine; blood pressure; hypertension; lacidipine.
Figures
Similar articles
-
Clinic blood pressure responses to two amlodipine salt formulations, adipate and besylate, in adult Korean patients with mild to moderate hypertension: a multicenter, randomized, double-blind, parallel-group, 8-week comparison.Clin Ther. 2005 Jun;27(6):728-39. doi: 10.1016/j.clinthera.2005.06.011. Clin Ther. 2005. PMID: 16117979 Clinical Trial.
-
Results of a phase III, 8-week, multicenter, prospective, randomized, double-blind, parallel-group clinical trial to assess the effects of amlodipine camsylate versus amlodipine besylate in Korean adults with mild to moderate hypertension.Clin Ther. 2007 Sep;29(9):1924-36. doi: 10.1016/j.clinthera.2007.09.018. Clin Ther. 2007. PMID: 18035192 Clinical Trial.
-
Comparison of efficacy and tolerability of amlodipine orotate versus amlodipine besylate in adult patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 8-week follow-up, noninferiority trial.Clin Ther. 2006 Apr;28(4):537-51. doi: 10.1016/j.clinthera.2006.04.008. Clin Ther. 2006. PMID: 16750465 Clinical Trial.
-
Efficacy and safety profiles of a new S(-)-amlodipine nicotinate formulation versus racemic amlodipine besylate in adult Korean patients with mild to moderate hypertension: an 8-week, multicenter, randomized, double-blind, double-dummy, parallel-group, phase III, noninferiority clinical trial.Clin Ther. 2008 May;30(5):845-57. doi: 10.1016/j.clinthera.2008.05.013. Clin Ther. 2008. PMID: 18555932 Clinical Trial.
-
Results of a multicenter, 8-week, parallel-group, randomized,double-blind, double-dummy, Phase III clinical trial to evaluate the efficacy and tolerability of amlodipine maleate versus amlodipine besylate in Korean patients with mild to moderate hypertension.Clin Ther. 2005 Apr;27(4):441-50. doi: 10.1016/j.clinthera.2005.04.001. Clin Ther. 2005. PMID: 15922817 Clinical Trial.
Cited by
-
Influence of G-protein β-Polypeptide 3 C825T Polymorphism on Antihypertensive Response to Telmisartan and Amlodipine in Chinese Patients.Chin Med J (Engl). 2016 Jan 5;129(1):8-14. doi: 10.4103/0366-6999.172548. Chin Med J (Engl). 2016. PMID: 26712426 Free PMC article.
-
Fimasartan versus perindopril with and without diuretics in the treatment of elderly patients with essential hypertension (Fimasartan in the Senior Subjects (FITNESS)): study protocol for a randomized controlled trial.Trials. 2019 Jul 1;20(1):389. doi: 10.1186/s13063-019-3466-5. Trials. 2019. PMID: 31262348 Free PMC article.
References
-
- Franklin S.S., Jacobs M.J., Wong N.D. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37:869–874. - PubMed
-
- Kannel W.B., Gordon T., Schwartz M.J. Systolic versus diastolic blood pressure and risk of coronary heart disease. The Framingham study. Am J Cardiol. 1971;27:335–346. - PubMed
-
- O'Donnell C.J., Ridker P.M., Glynn R.J. Hypertension and borderline isolated systolic hypertension increase risks of cardiovascular disease and mortality in male physicians. Circulation. 1997;95:1132–1137. - PubMed
-
- JAMA. 1991;265:3255–3264. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. - PubMed
-
- Staessen J.A., Fagard R., Thijs L. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

