ALCAM/CD166 is a TGF-β-responsive marker and functional regulator of prostate cancer metastasis to bone
- PMID: 24385212
- PMCID: PMC4149913
- DOI: 10.1158/0008-5472.CAN-13-1296
ALCAM/CD166 is a TGF-β-responsive marker and functional regulator of prostate cancer metastasis to bone
Abstract
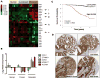
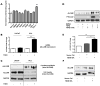
The dissemination of prostate cancer to bone is a common, incurable aspect of advanced disease. Prevention and treatment of this terminal phase of prostate cancer requires improved molecular understanding of the process as well as markers indicative of molecular progression. Through biochemical analyses and loss-of-function in vivo studies, we demonstrate that the cell adhesion molecule, activated leukocyte cell adhesion molecule (ALCAM), is actively shed from metastatic prostate cancer cells by the sheddase ADAM17 in response to TGF-β. Not only is this posttranslational modification of ALCAM a marker of prostate cancer progression, the molecule is also required for effective metastasis to bone. Biochemical analysis of prostate cancer cell lines reveals that ALCAM expression and shedding is elevated in response to TGF-β signaling. Both in vitro and in vivo shedding is mediated by ADAM17. Longitudinal analysis of circulating ALCAM in tumor-bearing mice revealed that shedding of tumor, but not host-derived ALCAM is elevated during growth of the cancer. Gene-specific knockdown of ALCAM in bone-metastatic PC3 cells greatly diminished both skeletal dissemination and tumor growth in bone. The reduced growth of ALCAM knockdown cells corresponded to an increase in apoptosis (caspase-3) and decreased proliferation (Ki67). Together, these data demonstrate that the ALCAM is both a functional regulator as well as marker of prostate cancer progression.
©2014 AACR
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
The authors have no competing interests.
Figures



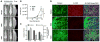

References
-
- Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. - PubMed
-
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. - PubMed
-
- van Kempen LC, Nelissen JM, Degen WG, Torensma R, Weidle UH, Bloemers HP, et al. Molecular basis for the homophilic activated leukocyte cell adhesion molecule (ALCAM)-ALCAM interaction. J Biol Chem. 2001;276:25783–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA163499/CA/NCI NIH HHS/United States
- S10 RR027631/RR/NCRR NIH HHS/United States
- CA143081/CA/NCI NIH HHS/United States
- K12 CA090625/CA/NCI NIH HHS/United States
- P01 CA040035/CA/NCI NIH HHS/United States
- T32 HL007751/HL/NHLBI NIH HHS/United States
- CA120711/CA/NCI NIH HHS/United States
- P50 CA098131/CA/NCI NIH HHS/United States
- I01 BX001957/BX/BLRD VA/United States
- F31 CA136228/CA/NCI NIH HHS/United States
- R01 CA143081/CA/NCI NIH HHS/United States
- CA136228/CA/NCI NIH HHS/United States
- K01 CA120711/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 GM008554/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

