Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity
- PMID: 24385344
- PMCID: PMC3928004
- DOI: 10.1093/cvr/cvt341
Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity
Abstract
Aims: Obesity is a risk factor for diabetes and cardiovascular diseases, with the incidence of these disorders becoming epidemic. Pathogenic responses to obesity have been ascribed to adipose tissue (AT) dysfunction that promotes bioactive mediator secretion from visceral AT and the initiation of pro-inflammatory events that induce oxidative stress and tissue dysfunction. Current understanding supports that suppressing pro-inflammatory and oxidative events promotes improved metabolic and cardiovascular function. In this regard, electrophilic nitro-fatty acids display pleiotropic anti-inflammatory signalling actions.
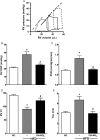
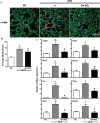
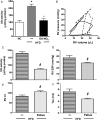
Methods and results: It was hypothesized that high-fat diet (HFD)-induced inflammatory and metabolic responses, manifested by loss of glucose tolerance and vascular dysfunction, would be attenuated by systemic administration of nitrooctadecenoic acid (OA-NO2). Male C57BL/6j mice subjected to a HFD for 20 weeks displayed increased adiposity, fasting glucose, and insulin levels, which led to glucose intolerance and pulmonary hypertension, characterized by increased right ventricular (RV) end-systolic pressure (RVESP) and pulmonary vascular resistance (PVR). This was associated with increased lung xanthine oxidoreductase (XO) activity, macrophage infiltration, and enhanced expression of pro-inflammatory cytokines. Left ventricular (LV) end-diastolic pressure remained unaltered, indicating that the HFD produces pulmonary vascular remodelling, rather than LV dysfunction and pulmonary venous hypertension. Administration of OA-NO2 for the final 6.5 weeks of HFD improved glucose tolerance and significantly attenuated HFD-induced RVESP, PVR, RV hypertrophy, lung XO activity, oxidative stress, and pro-inflammatory pulmonary cytokine levels.
Conclusions: These observations support that the pleiotropic signalling actions of electrophilic fatty acids represent a therapeutic strategy for limiting the complex pathogenic responses instigated by obesity.
Keywords: Inflammation; Nitro-fatty acid signalling; Obesity; Pulmonary hypertension; Xanthine Oxidase.
Figures



Comment in
-
Nitro-oleic acid and epoxy-oleic acid are not altered in obesity and type 2 diabetes.Cardiovasc Res. 2014 Jun 1;102(3):517-8. doi: 10.1093/cvr/cvu043. Epub 2014 Feb 21. Cardiovasc Res. 2014. PMID: 24562766 No abstract available.
References
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. - PubMed
-
- Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation. 2009;120:992–1007. - PubMed
-
- Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

