Clustering of tissue-specific sub-TADs accompanies the regulation of HoxA genes in developing limbs
- PMID: 24385922
- PMCID: PMC3873244
- DOI: 10.1371/journal.pgen.1004018
Clustering of tissue-specific sub-TADs accompanies the regulation of HoxA genes in developing limbs
Abstract
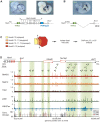
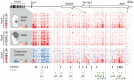
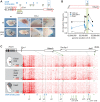
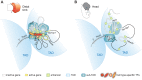
HoxA genes exhibit central roles during development and causal mutations have been found in several human syndromes including limb malformation. Despite their importance, information on how these genes are regulated is lacking. Here, we report on the first identification of bona fide transcriptional enhancers controlling HoxA genes in developing limbs and show that these enhancers are grouped into distinct topological domains at the sub-megabase scale (sub-TADs). We provide evidence that target genes and regulatory elements physically interact with each other through contacts between sub-TADs rather than by the formation of discreet "DNA loops". Interestingly, there is no obvious relationship between the functional domains of the enhancers within the limb and how they are partitioned among the topological domains, suggesting that sub-TAD formation does not rely on enhancer activity. Moreover, we show that suppressing the transcriptional activity of enhancers does not abrogate their contacts with HoxA genes. Based on these data, we propose a model whereby chromatin architecture defines the functional landscapes of enhancers. From an evolutionary standpoint, our data points to the convergent evolution of HoxA and HoxD regulation in the fin-to-limb transition, one of the major morphological innovations in vertebrates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Kmita M, Tarchini B, Zakany J, Logan M, Tabin CJ, et al. (2005) Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature 435: 1113–1116. - PubMed
-
- Medina-Martinez O, Bradley A, Ramirez-Solis R (2000) A large targeted deletion of Hoxb1-Hoxb9 produces a series of single-segment anterior homeotic transformations. Dev Biol 222: 71–83. - PubMed
-
- Suemori H, Noguchi S (2000) Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Dev Biol 220: 333–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

