Performance of a limiting-antigen avidity enzyme immunoassay for cross-sectional estimation of HIV incidence in the United States
- PMID: 24386116
- PMCID: PMC3873916
- DOI: 10.1371/journal.pone.0082772
Performance of a limiting-antigen avidity enzyme immunoassay for cross-sectional estimation of HIV incidence in the United States
Abstract
Background: A limiting antigen avidity enzyme immunoassay (HIV-1 LAg-Avidity assay) was recently developed for cross-sectional HIV incidence estimation. We evaluated the performance of the LAg-Avidity assay alone and in multi-assay algorithms (MAAs) that included other biomarkers.
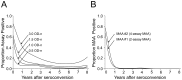
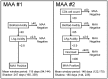
Methods and findings: Performance of testing algorithms was evaluated using 2,282 samples from individuals in the United States collected 1 month to >8 years after HIV seroconversion. The capacity of selected testing algorithms to accurately estimate incidence was evaluated in three longitudinal cohorts. When used in a single-assay format, the LAg-Avidity assay classified some individuals infected >5 years as assay positive and failed to provide reliable incidence estimates in cohorts that included individuals with long-term infections. We evaluated >500,000 testing algorithms, that included the LAg-Avidity assay alone and MAAs with other biomarkers (BED capture immunoassay [BED-CEIA], BioRad-Avidity assay, HIV viral load, CD4 cell count), varying the assays and assay cutoffs. We identified an optimized 2-assay MAA that included the LAg-Avidity and BioRad-Avidity assays, and an optimized 4-assay MAA that included those assays, as well as HIV viral load and CD4 cell count. The two optimized MAAs classified all 845 samples from individuals infected >5 years as MAA negative and estimated incidence within a year of sample collection. These two MAAs produced incidence estimates that were consistent with those from longitudinal follow-up of cohorts. A comparison of the laboratory assay costs of the MAAs was also performed, and we found that the costs associated with the optimal two assay MAA were substantially less than with the four assay MAA.
Conclusions: The LAg-Avidity assay did not perform well in a single-assay format, regardless of the assay cutoff. MAAs that include the LAg-Avidity and BioRad-Avidity assays, with or without viral load and CD4 cell count, provide accurate incidence estimates.
Conflict of interest statement
Figures
Comment in
-
Response to using incidence assays within the context of the recent infections testing algorithm.AIDS. 2014 Sep 10;28(14):2168. doi: 10.1097/QAD.0000000000000368. AIDS. 2014. PMID: 25265083 No abstract available.
References
-
- Brookmeyer R (2010) Measuring the HIV/AIDS epidemic: approaches and challenges. Epidemiol Rev 32: 26–37. - PubMed
-
- Busch MP, Pilcher CD, Mastro TD, Kaldor J, Vercauteren G, et al. (2010) Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 24: 2763–2771. - PubMed
-
- Brookmeyer R, Quinn T, Shepherd M, Mehendale S, Rodrigues J, et al. (1995) The AIDS epidemic in India: a new method for estimating current human immunodeficiency virus (HIV) incidence rates. Am J Epidemiol 142: 709–713. - PubMed
-
- Laeyendecker O, Brookmeyer R, Mullis CE, Donnell D, Lingappa J, et al. (2012) Specificity of four laboratory approaches for cross-sectional HIV incidence determination: analysis of samples from adults with known nonrecent HIV infection from five African countries. AIDS Res Hum Retroviruses 28: 1177–1183. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-45202/AI/NIAID NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- U01-AI35043/AI/NIAID NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- UM1-AI068619/AI/NIAID NIH HHS/United States
- R01 AI095068/AI/NIAID NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- R01-DA011602/DA/NIDA NIH HHS/United States
- R01-DA12568/DA/NIDA NIH HHS/United States
- R01-AA016893/AA/NIAAA NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- R01-DA-04334/DA/NIDA NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 DA036297/DA/NIDA NIH HHS/United States
- N01-AI35176/AI/NIAID NIH HHS/United States
- N01 AI045200/AI/NIAID NIH HHS/United States
- U01-AI35040/AI/NIAID NIH HHS/United States
- R01 DA004334/DA/NIDA NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- R01-AI095068/AI/NIAID NIH HHS/United States
- U01-AI35042/AI/NIAID NIH HHS/United States
- UL1-RR025005/RR/NCRR NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- U01-AI35041/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- U01-AI35039/AI/NIAID NIH HHS/United States
- R01 DA012568/DA/NIDA NIH HHS/United States
- UM1 AI068613/AI/NIAID NIH HHS/United States
- T32 GM008752/GM/NIGMS NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- N01-AI-45200/AI/NIAID NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- UM1-AI068613/AI/NIAID NIH HHS/United States
- UM1-AI068617/AI/NIAID NIH HHS/United States
- UM1 AI068617/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

