The influence of neuronal density and maturation on network activity of hippocampal cell cultures: a methodological study
- PMID: 24386305
- PMCID: PMC3873984
- DOI: 10.1371/journal.pone.0083899
The influence of neuronal density and maturation on network activity of hippocampal cell cultures: a methodological study
Abstract
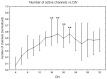
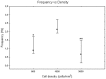
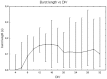
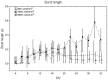
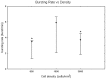
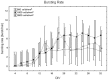
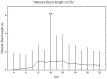
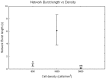
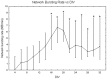
It is known that cell density influences the maturation process of in vitro neuronal networks. Neuronal cultures plated with different cell densities differ in number of synapses per neuron and thus in single neuron synaptic transmission, which results in a density-dependent neuronal network activity. Although many authors provided detailed information about the effects of cell density on neuronal culture activity, a dedicated report of density and age influence on neuronal hippocampal culture activity has not yet been reported. Therefore, this work aims at providing reference data to researchers that set up an experimental study on hippocampal neuronal cultures, helping in planning and decoding the experiments. In this work, we analysed the effects of both neuronal density and culture age on functional attributes of maturing hippocampal cultures. We characterized the electrophysiological activity of neuronal cultures seeded at three different cell densities, recording their spontaneous electrical activity over maturation by means of MicroElectrode Arrays (MEAs). We had gather data from 86 independent hippocampal cultures to achieve solid statistic results, considering the high culture-to-culture variability. Network activity was evaluated in terms of simple spiking, burst and network burst features. We observed that electrical descriptors were characterized by a functional peak during maturation, followed by a stable phase (for sparse and medium density cultures) or by a decrease phase (for high dense neuronal cultures). Moreover, 900 cells/mm(2) cultures showed characteristics suitable for long lasting experiments (e.g. chronic effect of drug treatments) while 1800 cells/mm(2) cultures should be preferred for experiments that require intense electrical activity (e.g. to evaluate the effect of inhibitory molecules). Finally, cell cultures at 3600 cells/mm(2) are more appropriate for experiments in which time saving is relevant (e.g. drug screenings). These results are intended to be a reference for the planning of in vitro neurophysiological and neuropharmacological experiments with MEAs.
Conflict of interest statement
Figures











References
-
- Morin F, Takamura Y, Tamiya E (2005) Investigating neuronal activity with planar microelectrode arrays: achievements and new perspectives. J Biosci Bioeng 100: 131–143. - PubMed
-
- Ben-Ari Y (2001) Developing networks play a similar melody. Trends Neurosci 24: 353–360. - PubMed
-
- Chiappalone M, Bove M, Vato A, Tedesco M, Martinoia S (2006) Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res 1093: 41–53. - PubMed
-
- Ichikawa M, Muramoto K, Kobayashi K, Kawahara M, Kuroda Y (1993) Formation and maturation of synapses in primary cultures of rat cerebral cortical cells: An electron microscopic study. Neurosci Res 16: 95–103. - PubMed
-
- Biffi E, Menegon A, Piraino F, Pedrocchi A, Fiore GB, et al. (2012) Validation of Long-Term Primary Neuronal Cultures and Network Activity Through the Integration of Reversibly Bonded Microbioreactors and MEA Substrates. Biotechnol Bioeng 109: 166–75. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

