Characterization of antigen-specific B cells using nominal antigen-coated flow-beads
- PMID: 24386360
- PMCID: PMC3875494
- DOI: 10.1371/journal.pone.0084273
Characterization of antigen-specific B cells using nominal antigen-coated flow-beads
Erratum in
- PLoS One. 2014;9(7):e102845. Ngono, Annie Elong [corrected to Elong Ngono, Annie]
Abstract
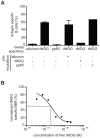
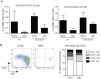
In order to characterize the reactivity of B cells against nominal antigens, a method based on the coupling of antigens onto the surface of fluorescent core polystyrene beads was developed. We first demonstrate that murine B cells with a human MOG-specific BCR are able to interact with MOG-coated beads and do not recognize beads coated with human albumin or pp65. B cells purified from human healthy volunteer blood or immunized individuals were tested for their ability to interact with various nominal antigens, including viral, vaccine, self and alloantigens, chosen for their usefulness in studying a variety of pathological processes. A substantial amount of B cells binding self-antigen MOG-coated beads can be detected in normal blood. Furthermore, greater frequencies of B cell against anti-Tetanic Toxin or anti-EBNA1 were observed in primed individuals. This method can reveal increased frequencies of anti-HLA committed B cells in patients with circulating anti-HLA antibodies compared to unsensitized patients and normal individuals. Of interest, those specific CD19 cells were preferentially identified within CD27(-)IgD(+) (i-e naïve) subset. These observations suggest that a broad range of medical situations could benefit from a tool that allows the detection, the quantification and the characterization of antigen-specific blood B cells.
Conflict of interest statement
Figures




References
-
- Brulhart L, Ciurea A, Finckh A, Notter A, Waldburger JM, et al. (2006) Efficacy of B cell depletion in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor agents: an open-label observational study. Annals of the Rheumatic Diseases 65: 1255–1257 10.1136/ard.2005.051169 - DOI - PMC - PubMed
-
- Frommer F, Heinen TJAJ, Wunderlich FT, Yogev N, Buch T, et al. (2008) Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. The Journal of Immunology 181: 5748–5759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

