Secretory activity is rapidly induced in stigmatic papillae by compatible pollen, but inhibited for self-incompatible pollen in the Brassicaceae
- PMID: 24386363
- PMCID: PMC3873414
- DOI: 10.1371/journal.pone.0084286
Secretory activity is rapidly induced in stigmatic papillae by compatible pollen, but inhibited for self-incompatible pollen in the Brassicaceae
Abstract
[In the Brassicaceae, targeted exocytosis to the stigmatic papillar plasma membrane under the compatible pollen grain is hypothesized to be essential for pollen hydration and pollen tube penetration. In contrast, polarized secretion is proposed to be inhibited in the stigmatic papillae during the rejection of self-incompatible pollen. Using transmission electron microscopy (TEM), we performed a detailed time-course of post-pollination events to view the cytological responses of the stigmatic papillae to compatible and self-incompatible pollinations. For compatible pollinations in Arabidopsis thaliana and Arabidopsis lyrata, vesicle secretion was observed at the stigmatic papillar plasma membrane under the pollen grain while Brassica napus stigmatic papillae appeared to use multivesicular bodies (MVBs) for secretion. Exo70A1, a component of the exocyst complex, has been previously implicated in the compatible pollen responses, and disruption of Exo70A1 in both A. thaliana and B. napus resulted in a loss of secretory vesicles/MVBs at the stigmatic papillar plasma membrane. Similarly, for self-incompatible pollinations, secretory vesicles/MVBs were absent from the stigmatic papillar plasma membrane in A. lyrata and B. napus; and furthermore, autophagy appeared to be induced to direct vesicles/MVBs to the vacuole for degradation. Thus, these findings support a model where the basal pollen recognition pathway in the stigmatic papilla promotes exocytosis to accept compatible pollen, and the basal pollen recognition pathway is overridden by the self-incompatibility pathway to prevent exocytosis and reject self-pollen.
Conflict of interest statement
Figures

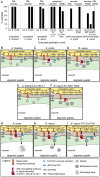



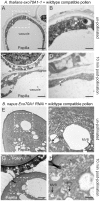

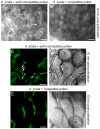

Comment in
-
Autophagy in the rejection of self-pollen in the mustard family.Autophagy. 2014;10(12):2379-80. doi: 10.4161/15548627.2014.981918. Autophagy. 2014. PMID: 25629934 Free PMC article.
References
-
- Chapman LA, Goring DR (2010) Pollen-pistil interactions regulating successful fertilization in the Brassicaceae. J Exp Bot 61: 1987–1999. - PubMed
-
- Dickinson H (1995) Dry stigmas, water and self-incompatibility in Brassica . Sex Plant Reprod 8: 1–10.
-
- Hiscock SJ, Allen AM (2008) Diverse cell signalling pathways regulate pollen-stigma interactions: the search for consensus. New Phytol 179: 286–317. - PubMed
-
- Mattson O, Knox RB, Heslopha J, Heslopha Y (1974) Protein pellicle of stigmatic papillae as a probable recognition site in incompatible reactions. Nature 247: 298–300.
-
- Stead AD, Roberts IN, Dickinson HG (1980) Pollen-stigma interactions in Brassica oleracea - The role of stigmatic proteins in pollen grain adhesion. J Cell Sci 42: 417–423. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

