Adherence to artemisinin-based combination therapy for the treatment of malaria: a systematic review of the evidence
- PMID: 24386988
- PMCID: PMC3893456
- DOI: 10.1186/1475-2875-13-7
Adherence to artemisinin-based combination therapy for the treatment of malaria: a systematic review of the evidence
Abstract
Background: Increasing access to and targeting of artemisinin-based combination therapy (ACT) is a key component of malaria control programmes. To maximize efficacy of ACT and ensure adequate treatment outcomes, patient and caregiver adherence to treatment guidelines is essential. This review summarizes the current evidence base on ACT adherence, including definitions, measurement methods, and associated factors.
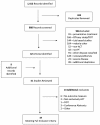
Methods: A systematic search of the published literature was undertaken in November 2012 and updated in April 2013. Bibliographies of manuscripts were also searched and additional references identified. Studies were included if they involved at least one form of ACT and reported an adherence measurement.
Results: The search yielded 1,412 records, 37 of which were found to measure adherence to ACT. Methods to measure adherence focused on self-report, pill counts and bioassays with varying definitions for adherence. Most studies only reported whether medication regimens were completed, but did not assess how the treatment was taken by the patient (i.e. timing, frequency and dose). Adherence data were available for four different ACT formulations: artemether-lumefantrine (AL) (range 39-100%), amodiaquine plus artesunate (AQ + AS) (range 48-94%), artesunate plus sulphadoxine-pyrimethamine (AS + SP) (range 39-75%) and artesunate plus mefloquine (AS + MQ) (range 77-95%). Association between demographic factors, such as age, gender, education and socio-economic status and adherence to ACT regimens was not consistent. Some evidence of positive association between adherence and patient age, caregiver education levels, drug preferences, health worker instructions, patient/caregiver knowledge and drug packaging were also observed.
Conclusions: This review highlights the weak evidence base on ACT adherence. Results suggest that ACT adherence levels varied substantially between study populations, but comparison between studies was challenging due to differences in study design, definitions, and methods used to measure adherence. Standardising methodologies for both self-report and bioassays used for evaluating adherence of different formulations across diverse contexts would improve the evidence base on ACT adherence and effectiveness; namely, specific and measurable definitions for adherence are needed for both methodologies. Additionally, further studies of the individual factors and barriers associated with non-adherence to ACT are needed in order to make informed policy choices and to improve the delivery of effective malaria treatment.
Figures

Similar articles
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
-
Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria.Cochrane Database Syst Rev. 2013 Oct 25;2013(10):CD008492. doi: 10.1002/14651858.CD008492.pub3. Cochrane Database Syst Rev. 2013. PMID: 24163021 Free PMC article.
-
Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria.Cochrane Database Syst Rev. 2014 Jan 20;2014(1):CD010927. doi: 10.1002/14651858.CD010927. Cochrane Database Syst Rev. 2014. PMID: 24443033 Free PMC article.
-
Subsidising artemisinin-based combination therapy in the private retail sector.Cochrane Database Syst Rev. 2016 Mar 9;3(3):CD009926. doi: 10.1002/14651858.CD009926.pub2. Cochrane Database Syst Rev. 2016. PMID: 26954551 Free PMC article.
-
Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD007768. doi: 10.1002/14651858.CD007768.pub3. Cochrane Database Syst Rev. 2014. PMID: 24777444 Free PMC article.
Cited by
-
Malaria policies versus practices, a reality check from Kinshasa, the capital of the Democratic Republic of Congo.BMC Public Health. 2015 Apr 10;15:352. doi: 10.1186/s12889-015-1670-0. BMC Public Health. 2015. PMID: 25885211 Free PMC article. Clinical Trial.
-
A randomized, double-blind, phase 2b study to investigate the efficacy, safety, tolerability and pharmacokinetics of a single-dose regimen of ferroquine with artefenomel in adults and children with uncomplicated Plasmodium falciparum malaria.Malar J. 2021 May 19;20(1):222. doi: 10.1186/s12936-021-03749-4. Malar J. 2021. PMID: 34011358 Free PMC article. Clinical Trial.
-
Malaria outbreak in Riaba district, Bioko Island: lessons learned.Malar J. 2020 Aug 3;19(1):277. doi: 10.1186/s12936-020-03347-w. Malar J. 2020. PMID: 32746919 Free PMC article.
-
Mobile phone short message service (SMS) as a malaria control tool: a quasi-experimental study.BMC Public Health. 2019 Aug 29;19(1):1193. doi: 10.1186/s12889-019-7336-6. BMC Public Health. 2019. PMID: 31464623 Free PMC article. Clinical Trial.
-
The gender-related variability in the pharmacokinetics and antiplasmodial activity of naphthoquine in rodents.Malar J. 2020 Feb 13;19(1):71. doi: 10.1186/s12936-020-3153-8. Malar J. 2020. PMID: 32054478 Free PMC article.
References
-
- WHO. World Malaria Report 2011. Geneva: World Health Organization; 2011.
-
- WHO. T3: Test. Treat. Track Initiative. Geneva: World Health Organization; 2012.
-
- WHO. Guidelines for the Treatment of Malaria. Second edn. Geneva: World Health Organization; 2010.
-
- Bosman A, Mendis KN. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am J Trop Med Hyg. 2007;77:193–197. - PubMed
-
- WHO. World Malaria Report 2009. Geneva: World Health Organization; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

