Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability
- PMID: 24387130
- PMCID: PMC3902061
- DOI: 10.1186/1754-6834-7-2
Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability
Abstract
Background: The genome of Pleurotus ostreatus, an important edible mushroom and a model ligninolytic organism of interest in lignocellulose biorefineries due to its ability to delignify agricultural wastes, was sequenced with the purpose of identifying and characterizing the enzymes responsible for lignin degradation.
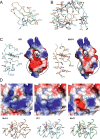
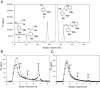
Results: Heterologous expression of the class II peroxidase genes, followed by kinetic studies, enabled their functional classification. The resulting inventory revealed the absence of lignin peroxidases (LiPs) and the presence of three versatile peroxidases (VPs) and six manganese peroxidases (MnPs), the crystal structures of two of them (VP1 and MnP4) were solved at 1.0 to 1.1 Å showing significant structural differences. Gene expansion supports the importance of both peroxidase types in the white-rot lifestyle of this fungus. Using a lignin model dimer and synthetic lignin, we showed that VP is able to degrade lignin. Moreover, the dual Mn-mediated and Mn-independent activity of P. ostreatus MnPs justifies their inclusion in a new peroxidase subfamily. The availability of the whole POD repertoire enabled investigation, at a biochemical level, of the existence of duplicated genes. Differences between isoenzymes are not limited to their kinetic constants. Surprising differences in their activity T50 and residual activity at both acidic and alkaline pH were observed. Directed mutagenesis and spectroscopic/structural information were combined to explain the catalytic and stability properties of the most interesting isoenzymes, and their evolutionary history was analyzed in the context of over 200 basidiomycete peroxidase sequences.
Conclusions: The analysis of the P. ostreatus genome shows a lignin-degrading system where the role generally played by LiP has been assumed by VP. Moreover, it enabled the first characterization of the complete set of peroxidase isoenzymes in a basidiomycete, revealing strong differences in stability properties and providing enzymes of biotechnological interest.
Figures





References
-
- Lavi I, Levinson D, Peri I, Tekoah Y, Hadar Y, Schwartz B. Chemical characterization, antiproliferative and antiadhesive properties of polysaccharides extracted from Pleurotus pulmonarius mycelium and fruiting bodies. Appl Microbiol Biotechnol. 2010;85:1977–1990. doi: 10.1007/s00253-009-2296-x. - DOI - PubMed
-
- Martínez AT, Camarero S, Guillén F, Gutiérrez A, Muñoz C, Varela E, Martínez MJ, Barrasa JM, Ruel K, Pelayo M. Progress in biopulping of non-woody materials: chemical, enzymatic and ultrastructural aspects of wheat-straw delignification with ligninolytic fungi from the genus Pleurotus. FEMS Microbiol Rev. 1994;13:265–274.
LinkOut - more resources
Full Text Sources
Other Literature Sources

