Host perception of jasmonates promotes infection by Fusarium oxysporum formae speciales that produce isoleucine- and leucine-conjugated jasmonates
- PMID: 24387225
- PMCID: PMC4211617
- DOI: 10.1111/mpp.12117
Host perception of jasmonates promotes infection by Fusarium oxysporum formae speciales that produce isoleucine- and leucine-conjugated jasmonates
Abstract
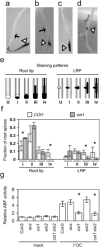
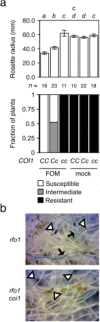
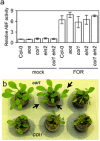
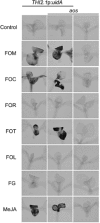
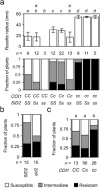
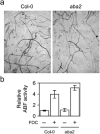
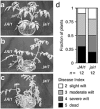
Three pathogenic forms, or formae speciales (f. spp.), of Fusarium oxysporum infect the roots of Arabidopsis thaliana below ground, instigating symptoms of wilt disease in leaves above ground. In previous reports, Arabidopsis mutants that are deficient in the biosynthesis of abscisic acid or salicylic acid or insensitive to ethylene or jasmonates exhibited either more or less wilt disease, than the wild-type, implicating the involvement of hormones in the normal host response to F. oxysporum. Our analysis of hormone-related mutants finds no evidence that endogenous hormones contribute to infection in roots. Mutants that are deficient in abscisic acid and insensitive to ethylene show no less infection than the wild-type, although they exhibit less disease. Whether a mutant that is insensitive to jasmonates affects infection depends on which forma specialis (f. sp.) is infecting the roots. Insensitivity to jasmonates suppresses infection by F. oxysporum f. sp. conglutinans and F. oxysporum f. sp. matthioli, which produce isoleucine- and leucine-conjugated jasmonate (JA-Ile/Leu), respectively, in culture filtrates, whereas insensitivity to jasmonates has no effect on infection by F. oxysporum f. sp. raphani, which produces no detectable JA-Ile/Leu. Furthermore, insensitivity to jasmonates has no effect on wilt disease of tomato, and the tomato pathogen F. oxysporum f. sp. lycopersici produces no detectable jasmonates. Thus, some, but not all, F. oxysporum pathogens appear to utilize jasmonates as effectors, promoting infection in roots and/or the development of symptoms in shoots. Only when the infection of roots is promoted by jasmonates is wilt disease enhanced in a mutant deficient in salicylic acid biosynthesis.
Keywords: Arabidopsis thaliana; COI1; Fusarium oxysporum; Fusarium wilt; jasmonate.
© 2014 BSPP AND JOHN WILEY & SONS LTD.
Figures


References
-
- Aboul‐Soud, M.A. , Yun, B.W. , Harrier, L.A. and Loake, G.J. (2004) Transformation of Fusarium oxysporum by particle bombardment and characterisation of the resulting transformants expressing a GFP transgene. Mycopathologia, 158, 475–482. - PubMed
-
- Alonso, J.M. , Hirayama, T. , Roman, G. , Nourizadeh, S. and Ecker, J.R. (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science, 284, 2148–2152. - PubMed
-
- Anderson, J.P. , Badruzsaufari, E. , Schenk, P.M. , Manners, J.M. , Desmond, O.J. , Ehlert, C. , Maclean, D.J. , Ebert, P.R. and Kazan, K. (2004) Antagonistic interaction between abscisic acid and jasmonate ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell, 16, 3460–3479. - PMC - PubMed
-
- Beckman, C.H. (1987) The Nature of Wilt Diseases of Plants. St. Paul, MN: American Phytopathological Society.
-
- Beckman, C.H. and Roberts, E.M. (1995) On the nature and genetic basis for resistance and tolerance to Fungal wilt diseases of plants. Adv. Bot. Res. 21, 35–77.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

