T1-mapping in the heart: accuracy and precision
- PMID: 24387626
- PMCID: PMC3927683
- DOI: 10.1186/1532-429X-16-2
T1-mapping in the heart: accuracy and precision
Abstract
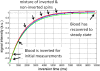
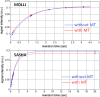
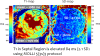
The longitudinal relaxation time constant (T1) of the myocardium is altered in various disease states due to increased water content or other changes to the local molecular environment. Changes in both native T1 and T1 following administration of gadolinium (Gd) based contrast agents are considered important biomarkers and multiple methods have been suggested for quantifying myocardial T1 in vivo. Characterization of the native T1 of myocardial tissue may be used to detect and assess various cardiomyopathies while measurement of T1 with extracellular Gd based contrast agents provides additional information about the extracellular volume (ECV) fraction. The latter is particularly valuable for more diffuse diseases that are more challenging to detect using conventional late gadolinium enhancement (LGE). Both T1 and ECV measures have been shown to have important prognostic significance. T1-mapping has the potential to detect and quantify diffuse fibrosis at an early stage provided that the measurements have adequate reproducibility. Inversion recovery methods such as MOLLI have excellent precision and are highly reproducible when using tightly controlled protocols. The MOLLI method is widely available and is relatively mature. The accuracy of inversion recovery techniques is affected significantly by magnetization transfer (MT). Despite this, the estimate of apparent T1 using inversion recovery is a sensitive measure, which has been demonstrated to be a useful tool in characterizing tissue and discriminating disease. Saturation recovery methods have the potential to provide a more accurate measurement of T1 that is less sensitive to MT as well as other factors. Saturation recovery techniques are, however, noisier and somewhat more artifact prone and have not demonstrated the same level of reproducibility at this point in time.This review article focuses on the technical aspects of key T1-mapping methods and imaging protocols and describes their limitations including the factors that influence their accuracy, precision, and reproducibility.
Figures













References
-
- Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. doi: 10.1186/1532-429X-15-92. - DOI - PMC - PubMed
-
- Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126(10):1206–16. doi: 10.1161/CIRCULATIONAHA.111.089409. - DOI - PMC - PubMed
-
- Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2013. doi: 10.1093/eurheartj/eht193. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

