Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis
- PMID: 24387680
- PMCID: PMC4056005
- DOI: 10.1186/cc13176
Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis
Abstract
Introduction: A major pathophysiologic mechanism in sepsis is impaired host immunity which results in failure to eradicate invading pathogens and increased susceptibility to secondary infections. Although many immunosuppressive mechanisms exist, increased expression of the inhibitory receptor programmed cell death 1 (PD-1) and its ligand (PD-L1) are thought to play key roles. The newly recognized phenomenon of T cell exhaustion is mediated in part by PD-1 effects on T cells. This study tested the ability of anti-PD-1 and anti-PD-L1 antibodies to prevent apoptosis and improve lymphocyte function in septic patients.
Methods: Blood was obtained from 43 septic and 15 non-septic critically-ill patients. Effects of anti-PD-1, anti-PD-L1, or isotype-control antibody on lymphocyte apoptosis and interferon gamma (IFN-γ) and interleukin-2 (IL-2) production were quantitated by flow cytometry.
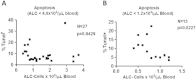
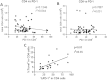
Results: Lymphocytes from septic patients produced decreased IFN-γ and IL-2 and had increased CD8 T cell expression of PD-1 and decreased PD-L1 expression compared to non-septic patients (P<0.05). Monocytes from septic patients had increased PD-L1 and decreased HLA-DR expression compared to non-septic patients (P<0.01). CD8 T cell expression of PD-1 increased over time in ICU as PD-L1, IFN-γ, and IL2 decreased. In addition, donors with the highest CD8 PD-1 expression together with the lowest CD8 PD-L1 expression also had lower levels of HLA-DR expression in monocytes, and an increased rate of secondary infections, suggestive of a more immune exhausted phenotype. Treatment of cells from septic patients with anti-PD-1 or anti-PD-L1 antibody decreased apoptosis and increased IFN-γ and IL-2 production in septic patients; (P<0.01). The percentage of CD4 T cells that were PD-1 positive correlated with the degree of cellular apoptosis (P<0.01).
Conclusions: In vitro blockade of the PD-1:PD-L1 pathway decreases apoptosis and improves immune cell function in septic patients. The current results together with multiple positive studies of anti-PD-1 and anti-PD-L1 in animal models of bacterial and fungal infections and the relative safety profile of anti-PD-1/anti-PD-L1 in human oncology trials to date strongly support the initiation of clinical trials testing these antibodies in sepsis, a disorder with a high mortality.
Figures







References
-
- Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

