A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone
- PMID: 24388382
- PMCID: PMC3905838
- DOI: 10.1016/j.biomaterials.2013.11.050
A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone
Abstract
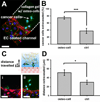
Cancer metastases arise following extravasation of circulating tumor cells with certain tumors exhibiting high organ specificity. Here, we developed a 3D microfluidic model to analyze the specificity of human breast cancer metastases to bone, recreating a vascularized osteo-cell conditioned microenvironment with human osteo-differentiated bone marrow-derived mesenchymal stem cells and endothelial cells. The tri-culture system allowed us to study the transendothelial migration of highly metastatic breast cancer cells and to monitor their behavior within the bone-like matrix. Extravasation, quantified 24 h after cancer cell injection, was significantly higher in the osteo-cell conditioned microenvironment compared to collagen gel-only matrices (77.5 ± 3.7% vs. 37.6 ± 7.3%), and the migration distance was also significantly greater (50.8 ± 6.2 μm vs. 31.8 ± 5.0 μm). Extravasated cells proliferated to form micrometastases of various sizes containing 4 to more than 60 cells by day 5. We demonstrated that the breast cancer cell receptor CXCR2 and the bone-secreted chemokine CXCL5 play a major role in the extravasation process, influencing extravasation rate and traveled distance. Our study provides novel 3D in vitro quantitative data on extravasation and micrometastasis generation of breast cancer cells within a bone-like microenvironment and demonstrates the potential value of microfluidic systems to better understand cancer biology and screen for new therapeutics.
Keywords: Bone; Breast cancer; Extravasation; Hydrogel; Metastasis; Microfluidics.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis.Nat Commun. 2019 Sep 27;10(1):4404. doi: 10.1038/s41467-019-12108-6. Nat Commun. 2019. PMID: 31562303 Free PMC article.
-
Microfluidic platform for studying osteocyte mechanoregulation of breast cancer bone metastasis.Integr Biol (Camb). 2019 Apr 1;11(4):119-129. doi: 10.1093/intbio/zyz008. Integr Biol (Camb). 2019. PMID: 31125041
-
Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor.Cancer Lett. 2011 Sep 1;308(1):91-9. doi: 10.1016/j.canlet.2011.04.018. Epub 2011 May 23. Cancer Lett. 2011. PMID: 21601983 Free PMC article.
-
Molecular insights into the interplay between adiposity, breast cancer and bone metastasis.Clin Exp Metastasis. 2021 Apr;38(2):119-138. doi: 10.1007/s10585-021-10076-0. Epub 2021 Feb 16. Clin Exp Metastasis. 2021. PMID: 33591548 Review.
-
Implications of Hypoxia in Breast Cancer Metastasis to Bone.Int J Mol Sci. 2016 Sep 30;17(10):1669. doi: 10.3390/ijms17101669. Int J Mol Sci. 2016. PMID: 27706047 Free PMC article. Review.
Cited by
-
Contribution of Osteoblast and Osteoclast Supernatants to Bone Formation: Determination Using a Novel Microfluidic Chip.Int J Mol Sci. 2024 Jun 15;25(12):6605. doi: 10.3390/ijms25126605. Int J Mol Sci. 2024. PMID: 38928310 Free PMC article.
-
Organ on Chip Technology to Model Cancer Growth and Metastasis.Bioengineering (Basel). 2022 Jan 11;9(1):28. doi: 10.3390/bioengineering9010028. Bioengineering (Basel). 2022. PMID: 35049737 Free PMC article. Review.
-
Mimicking Molecular Pathways in the Design of Smart Hydrogels for the Design of Vascularized Engineered Tissues.Int J Mol Sci. 2023 Aug 1;24(15):12314. doi: 10.3390/ijms241512314. Int J Mol Sci. 2023. PMID: 37569691 Free PMC article. Review.
-
Joint-on-chip platforms: entering a new era of in vitro models for arthritis.Nat Rev Rheumatol. 2022 Apr;18(4):217-231. doi: 10.1038/s41584-021-00736-6. Epub 2022 Jan 20. Nat Rev Rheumatol. 2022. PMID: 35058618 Review.
-
Evaluating the Role of IL-1β in Transmigration of Triple Negative Breast Cancer Cells Across the Brain Endothelium.Cell Mol Bioeng. 2021 Oct 26;15(1):99-114. doi: 10.1007/s12195-021-00710-y. eCollection 2022 Feb. Cell Mol Bioeng. 2021. PMID: 35096187 Free PMC article.
References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. - PubMed
-
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
-
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. - PubMed
-
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

