Spatial control of adult stem cell fate using nanotopographic cues
- PMID: 24388388
- PMCID: PMC4459500
- DOI: 10.1016/j.biomaterials.2013.11.037
Spatial control of adult stem cell fate using nanotopographic cues
Abstract
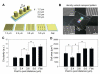
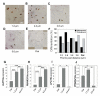
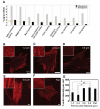
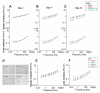
Adult stem cells hold great promise as a source of diverse terminally differentiated cell types for tissue engineering applications. However, due to the complexity of chemical and mechanical cues specifying differentiation outcomes, development of arbitrarily complex geometric and structural arrangements of cells, adopting multiple fates from the same initial stem cell population, has been difficult. Here, we show that the topography of the cell adhesion substratum can be an instructive cue to adult stem cells and topographical variations can strongly bias the differentiation outcome of the cells towards adipocyte or osteocyte fates. Switches in cell fate decision from adipogenic to osteogenic lineages were accompanied by changes in cytoskeletal stiffness, spanning a considerable range in the cell softness/rigidity spectrum. Our findings suggest that human mesenchymal stem cells (hMSC) can respond to the varying density of nanotopographical cues by regulating their internal cytoskeletal network and use these mechanical changes to guide them toward making cell fate decisions. We used this finding to design a complex two-dimensional pattern of co-localized cells preferentially adopting two alternative fates, thus paving the road for designing and building more complex tissue constructs with diverse biomedical applications.
Keywords: Adipogenesis; Capillary force lithography; Differentiation; Human mesenchymal stem cells; Nanotopography; Osteogenesis.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Palpant NJ, Murry CE. Regenerative medicine: reprogramming the injured heart. Nature. 2012;485:585–6. - PubMed
-
- Delli Carri A, Onorati M, Castiglioni V, Faedo A, Camnasio S, Toselli M, et al. Human pluripotent stem cell differentiation into authentic striatal projection neurons. Stem Cell Rev. 2013;9:461–74. - PubMed
-
- Marban E, Malliaras K. Mixed results for bone marrow-derived cell therapy for ischemic heart disease. JAMA. 2012;308:2405–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

