Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria
- PMID: 24388751
- PMCID: PMC4105362
- DOI: 10.1016/j.celrep.2013.12.014
Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria
Abstract
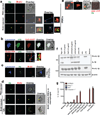
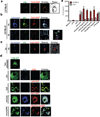
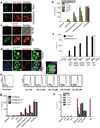
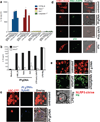
Hemozoin (Hz) is the crystalline detoxification product of hemoglobin in Plasmodium-infected erythrocytes. We previously proposed that Hz can carry plasmodial DNA into a subcellular compartment that is accessible to Toll-like receptor 9 (TLR9), inducing an inflammatory signal. Hz also activates the NLRP3 inflammasome in primed cells. We found that Hz appears to colocalize with DNA in infected erythrocytes, even before RBC rupture or phagolysosomal digestion. Using synthetic Hz coated in vitro with plasmodial genomic DNA (gDNA) or CpG oligodeoxynucleotides, we observed that DNA-complexed Hz induced TLR9 translocation, providing a priming and an activation signal for inflammasomes. After phagocytosis, Hz and DNA dissociate. Hz subsequently induces phagolysosomal destabilization, allowing phagolysosomal contents access to the cytosol, where DNA receptors become activated. Similar observations were made with Plasmodium-infected RBCs. Finally, infected erythrocytes activated both the NLRP3 and AIM2 inflammasomes. These observations suggest that Hz and DNA work together to induce systemic inflammation during malaria.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Adachi K, Tsutsui H, Kashiwamura S, Seki E, Nakano H, Takeuchi O, Takeda K, Okumura K, Van Kaer L, Okamura H, et al. Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J Immunol. 2001;167:5928–5934. - PubMed
-
- Ashong JO, Blench IP, Warhurst DC. The composition of haemozoin from Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1989;83:167–172. - PubMed
-
- Barrera V, Skorokhod OA, Baci D, Gremo G, Arese P, Schwarzer E. Host fibrinogen stably bound to hemozoin rapidly activates monocytes via TLR-4 and CD11b/CD18-integrin: a new paradigm of hemozoin action. Blood. 2011;117:5674–5682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

