AMPK: a cellular metabolic and redox sensor. A minireview
- PMID: 24389195
- PMCID: PMC4101001
- DOI: 10.2741/4218
AMPK: a cellular metabolic and redox sensor. A minireview
Abstract
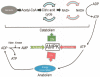
AMPK is a serine/threonine kinase that is found in all eukaryotes and is ubiquitously expressed in all organ systems. Once activated, AMPK stimulates hepatic fatty acid oxidation and ketogenesis, inhibits cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibits adipocyte lipolysis and lipogenesis, stimulates skeletal muscle fatty acid oxidation and muscle glucose uptake, and modulates insulin secretion by the pancreas. Thus its importance in many critical cellular processes is well established. For cells it is critical that energy supply and demand are closely matched. AMPK is recognized as a critical integrator of this balance. It is known to be allosterically activated by an increased AMP:ATP ratio. Activation of the kinase switches on catabolic pathways while switching off anabolic ones. It also acts as a redox sensor in endothelial cells where oxidative stress can disturb NO signaling. Abnormal NO signaling leads to disturbed vasodilatory responses. By inhibiting the formation of reactive oxygen species in the endothelium, AMPK can optimize the redox balance in the vasculature. Here, we review the role of AMPK in the cell.
Figures








References
-
- Aruksakunwong O, Wittayanarakul K, Sompornpisut P, Sanghiran V, Parasuk V, Hannongbua S. Structural and dynamical properties of different protonated states of mutant HIV-1 protease complexed with the saquinavir inhibitor studied by molecular dynamics simulations. J Mol Graph Model. 2006;25(3):324–32. - PubMed
-
- Neuhuber WL. Lung sensors: complex functions require complex structures. Am J Respir Cell Mol Biol. 2003;28(3):265–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

