YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors
- PMID: 24389730
- PMCID: PMC4222524
- DOI: 10.1530/ERC-13-0339
YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors
Abstract
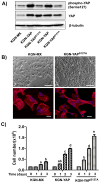
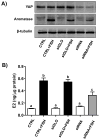
The Hippo signaling pathway has been implicated as a conserved regulator of organ size in both Drosophila and mammals. Yes-associated protein (YAP), the central component of the Hippo signaling cascade, functions as an oncogene in several malignancies. Ovarian granulosa cell tumors (GCT) are characterized by enlargement of the ovary, excess production of estrogen, a high frequency of recurrence, and the potential for malignancy and metastasis. Whether the Hippo pathway plays a role in the pathogenesis of GCT is unknown. This study was conducted to examine the expression of YAP in human adult GCTs and to determine the role of YAP in the proliferation and steroidogenesis of GCT cells. Compared with age-matched normal human ovaries, GCT tissues exhibited higher levels of YAP expression. YAP protein was predominantly expressed in the nucleus of tumor cells, whereas the non-tumor ovarian stromal cells expressed very low levels of YAP. YAP was also expressed in cultured primary human granulosa cells and in KGN and COV434 GCT cell lines. siRNA-mediated knockdown of YAP in KGN cells resulted in a significant reduction in cell proliferation (P<0.001). Conversely, overexpression of wild type YAP or a constitutively active YAP (YAP1) mutant resulted in a significant increase in KGN cell proliferation and migration. Moreover, YAP knockdown reduced FSH-induced aromatase (CYP19A1) protein expression and estrogen production in KGN cells. These results demonstrate that YAP plays an important role in the regulation of GCT cell proliferation, migration, and steroidogenesis. Targeting the Hippo/YAP pathway may provide a novel therapeutic approach for GCT.
Keywords: Hippo/YAP pathway; cell proliferation; granulosa cell tumor; migration; steroidogenesis.
Figures




References
-
- Aboud E. A review of granulosa cell tumours and thecomas of the ovary. Arch Gynecol Obstet. 1997;259:161–165. - PubMed
-
- Adashi EY, Hsueh AJ. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem. 1982;257:6077–6083. - PubMed
-
- Al-Agha OM, Huwait HF, Chow C, Yang W, Senz J, Kalloger SE, Huntsman DG, Young RH, Gilks CB. FOXL2 is a sensitive and specific marker for sex cord-stromal tumors of the ovary. Am J Surg Pathol. 2011;35:484–494. - PubMed
-
- Alhilli MM, Long HJ, Podratz KC, Bakkum-Gamez JN. Aromatase inhibitors in the treatment of recurrent ovarian granulosa cell tumors: brief report and review of the literature. J Obstet Gyaecol Res. 2012;38:340–344. - PubMed
-
- Amsterdam A, Selvaraj N. Control of differentiation, transformation, and apoptosis in granulosa cells by oncogenes, oncoviruses, and tumor suppressor genes. Endocr Rev. 1997;18:435–461. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

