Predicting the pathologic response of locally advanced rectal cancer to neoadjuvant concurrent chemoradiation using enzyme-linked immunosorbent assays (ELISAs) for biomarkers
- PMID: 24390211
- PMCID: PMC11823549
- DOI: 10.1007/s00432-013-1578-y
Predicting the pathologic response of locally advanced rectal cancer to neoadjuvant concurrent chemoradiation using enzyme-linked immunosorbent assays (ELISAs) for biomarkers
Abstract
Purpose: To investigate the role of biomarkers including serum tissue inhibitor of metalloproteinases-1 (TIMP-1), urokinase plasminogen activator receptor, vascular endothelial growth factor, and epidermal growth factor receptor in predicting pathologic response to neoadjuvant chemoradiation (NACRT) for rectal cancer.
Methods: Between 2007 and 2009, 50 clinical TNM stage II or III patients were analyzed in this prospective study. Pre- and post-NACRT serum levels of biomarkers were assessed using ELISAs. The primary and secondary endpoints were pathologic complete response (pCR) and Mandard regression grade (MRG).
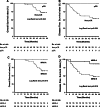
Results: The pCR was reported in 5 patients (10.0%). According to the MRG, fifteen patients (30.0%) were divided into group A (Grade I-II), the others in group B (Grade III-V). On univariate analysis, post-NACRT TIMP-1 showed notable significance with pCR (P = 0.092) and significant correlation with MRG group A (P = 0.003). Post-NACRT TIMP-1 ≤ 204.5 ng/mL as cut-off value by ROC curve was associated with more pCR and MRG group A patients (P = 0.016 and 0.002). Interval between NACRT and surgery was related to pCR with approached trend levels of significance (P = 0.05) and to MRG group A significantly on univariate analysis of clinical factors (P = 0.031). On multivariate analysis, post-NACRT TIMP-1 was not significantly related to pCR (P = 0.187), while it was significantly associated with MRG (P = 0.009). Among clinical responders, post-NACRT TIMP-1 level ≤ 204.5 ng/mL was significantly associated with pCR (P = 0.021) and MRG group A (P = 0.003).
Conclusions: Post-NACRT serum TIMP-1 could be used as a predictive marker of pathologic response to NACRT in rectal cancer, even in patients with clinical response.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures

Similar articles
-
Smoking and Elevated Preneoadjuvant Chemoradiotherapy Serum Carcinoembryonic Antigen Levels Are Associated With High Tumor Regression Grade and Poor Survival in Patients With Locally Advanced Rectal Cancer.Kaohsiung J Med Sci. 2025 Jun;41(6):e70008. doi: 10.1002/kjm2.70008. Epub 2025 Mar 13. Kaohsiung J Med Sci. 2025. PMID: 40078099 Free PMC article.
-
Evaluation of pre-treatment F-18 FDG PET/CT according to Mandard classification in locally advanced rectal cancer patients undergoing neoadjuvant chemoradiotherapy.BMC Cancer. 2025 Aug 4;25(1):1262. doi: 10.1186/s12885-025-14659-y. BMC Cancer. 2025. PMID: 40760425 Free PMC article.
-
Vascular calcification and response to neoadjuvant therapy in locally advanced rectal cancer: an exploratory study.J Cancer Res Clin Oncol. 2021 Nov;147(11):3409-3420. doi: 10.1007/s00432-021-03570-1. Epub 2021 Mar 12. J Cancer Res Clin Oncol. 2021. PMID: 33710416 Free PMC article.
-
Response prediction for neoadjuvant treatment in locally advanced rectal cancer patients-improvement in decision-making: A systematic review.Eur J Surg Oncol. 2025 Jul;51(7):109463. doi: 10.1016/j.ejso.2024.109463. Epub 2024 Nov 15. Eur J Surg Oncol. 2025. PMID: 39562260
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2015 Apr 7;(4):CD010260. doi: 10.1002/14651858.CD010260.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Aug 22;8:CD010260. doi: 10.1002/14651858.CD010260.pub3. PMID: 25847525 Updated.
References
-
- Birgisson H, Nielsen HJ, Christensen IJ, Glimelius B, Brunner N (2010) Preoperative plasma TIMP-1 is an independent prognostic indicator in patients with primary colorectal cancer: a prospective validation study. Eur J Cancer 46:3323–3331. doi:10.1016/j.ejca.2010.06.009 - PubMed
-
- Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S, Mattia FO, Friso ML, Genovesi D, Vidali C, Gambacorta MA, Buffoli A, Lupattelli M, Favretto MS, La Torre G (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72:99–107. doi:10.1016/j.ijrobp.2007.12.019 - PubMed
-
- Chan AK, Wong A, Jenken D, Heine J, Buie D, Johnson D (2005) Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys 61:665–677. doi:10.1016/j.ijrobp.2004.06.206 - PubMed
-
- Edge SB, American Joint Committee on Cancer (AJCC), American Cancer Society (2010) AJCC cancer staging handbook: from the AJCC cancer staging manual, 7th edn. Springer, New York
-
- Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP (1999) Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 17:2396 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

