Tyrosine kinase receptors as molecular targets in pheochromocytomas and paragangliomas
- PMID: 24390213
- PMCID: PMC4977182
- DOI: 10.1038/modpathol.2013.233
Tyrosine kinase receptors as molecular targets in pheochromocytomas and paragangliomas
Abstract
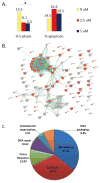
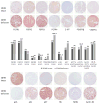
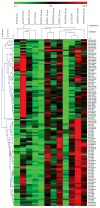
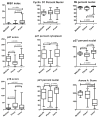
Pheochromocytomas and paragangliomas are neuroendocrine tumors shown to be responsive to multitargeted tyrosine kinase inhibitor (TKI) treatment. Despite growing knowledge regarding their genetic basis, the ability to predict behavior in these tumors remains challenging. There is also limited knowledge of their tyrosine kinase receptor expression and whether the clinical response observed to the TKI sunitinib relates only to its anti-angiogenic properties or also due to a direct effect on tumor cells. To answer these questions, an in vitro model of sunitinib treatment of a pheochromocytoma cell line was created. Sunitinib targets (VEGFRs, PDGFRs, and C-KIT), FGFRs, and cell cycle regulatory proteins were investigated in human tissue microarrays. SDHB immunohistochemistry was used as a surrogate marker for the presence of succinate dehydrogenase mutations. The FGFR4 G388R single nucleotide polymorphism was also investigated. Sunitinib treatment in vitro decreases cell proliferation mainly by targeting cell cycle, DNA metabolism, and cell organization genes. FGFR1, -2, and -4, VEGFR2, PDGFRα, and p16 were overexpressed in primary human pheochromocytomas and paragangliomas. Discordant results were observed for VEGFR1, p27, and p21 overexpressed in paragangliomas but underexpressed in pheochromocytomas; PDGFRβ, Rb, and Cyclin D1 overexpressed in paragangliomas only; and FGFR3 overexpressed in pheochromocytomas and underexpressed in paragangliomas. Low expression of C-KIT, p53, and Aurora kinase A and B was observed. Nuclear FGFR2 expression was associated with increased risk of metastasis (odds ratio (OR)=7.61, P=0.008), as was membranous PDGFRα (OR=13.71, P=0.015), membranous VEGFR1 (OR=8.01, P=0.037), nuclear MIB1 (OR=1.26, P=0.008), and cytoplasmic p27 (OR=1.037, P=0.030). FGFR3, VEGFR2, and C-KIT levels were associated with decreased risk of metastasis. We provide new insights into the mechanistic actions of sunitinib in pheochromocytomas and paragangliomas, and support current evidence that multitargeted TKIs might be a suitable treatment alternative for these tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Both sunitinib and sorafenib are effective treatments for pheochromocytoma in a xenograft model.Cancer Lett. 2014 Oct 1;352(2):236-44. doi: 10.1016/j.canlet.2014.07.005. Epub 2014 Jul 9. Cancer Lett. 2014. PMID: 25016061
-
Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248.Mol Cancer Ther. 2006 May;5(5):1280-9. doi: 10.1158/1535-7163.MCT-03-0156. Mol Cancer Ther. 2006. PMID: 16731761
-
A Guide to Pheochromocytomas and Paragangliomas.Surg Pathol Clin. 2019 Dec;12(4):951-965. doi: 10.1016/j.path.2019.08.009. Epub 2019 Sep 28. Surg Pathol Clin. 2019. PMID: 31672301 Free PMC article. Review.
-
Inactivation of SDH and FH cause loss of 5hmC and increased H3K9me3 in paraganglioma/pheochromocytoma and smooth muscle tumors.Oncotarget. 2015 Nov 17;6(36):38777-88. doi: 10.18632/oncotarget.6091. Oncotarget. 2015. PMID: 26472283 Free PMC article.
-
Pheochromocytomas and Paragangliomas: An Update on Recent Molecular Genetic Advances and Criteria for Malignancy.Adv Anat Pathol. 2015 Sep;22(5):283-93. doi: 10.1097/PAP.0000000000000086. Adv Anat Pathol. 2015. PMID: 26262510 Review.
Cited by
-
A Multi-Institutional Retrospective Analysis of Toceranib Phosphate for Presumed or Confirmed Canine Aortic Body Chemodectomas.Front Vet Sci. 2021 Feb 5;8:635057. doi: 10.3389/fvets.2021.635057. eCollection 2021. Front Vet Sci. 2021. PMID: 33614771 Free PMC article.
-
Paragangliomas arise through an autonomous vasculo-angio-neurogenic program inhibited by imatinib.Acta Neuropathol. 2018 May;135(5):779-798. doi: 10.1007/s00401-017-1799-2. Epub 2018 Jan 5. Acta Neuropathol. 2018. PMID: 29305721 Free PMC article.
-
Sunitinib Treatment for Advanced Paraganglioma: Case Report of a Novel SDHD Gene Mutation Variant and Systematic Review of the Literature.Front Oncol. 2021 Jun 17;11:677983. doi: 10.3389/fonc.2021.677983. eCollection 2021. Front Oncol. 2021. PMID: 34221997 Free PMC article.
-
Malignant paraganglioma of the posterior mediastinum: A case report with genetic analysis.Mol Clin Oncol. 2019 Jan;10(1):10-16. doi: 10.3892/mco.2018.1758. Epub 2018 Nov 8. Mol Clin Oncol. 2019. PMID: 30655972 Free PMC article.
-
Programmed cell death ligands expression in phaeochromocytomas and paragangliomas: Relationship with the hypoxic response, immune evasion and malignant behavior.Oncoimmunology. 2017 Aug 4;6(11):e1358332. doi: 10.1080/2162402X.2017.1358332. eCollection 2017. Oncoimmunology. 2017. PMID: 29147618 Free PMC article.
References
-
- Lloyd RV, Tischler AS, Kimura N, McNicol AM, Young WF., Jr . Adrenal tumors: introduction. In: DeLellis RA, Lloyd RV, Heitz PU, Eng C, editors. Pathology and genetics of tumours of endocrine organs. World Health Organization classification of tumours. IARC Press; Lyon, France: 2004. pp. 137–138.
-
- Gimenez-Roqueplo AP, Tischler AS. Pheochromocytoma and Paraganglioma: Progress on all fronts. Endocr Pathol. 2012;23:1–3. - PubMed
-
- Clarke MR, Weyant RJ, Watson CG, Carty SE. Prognostic markers in pheochromocytoma. Hum Pathol. 1998;29:522–6. - PubMed
-
- August C, August K, Schroeder S, et al. CGH and CD 44/MIB-1 immunohistochemistry are helpful to distinguish metastasized from nonmetastasized sporadic pheochromocytomas. Mod Pathol. 2004;17:1119–28. - PubMed
-
- Ayala-Ramirez M, Feng L, Johnson MM, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: Primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab. 2011;96:717–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

