Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide
- PMID: 24390325
- PMCID: PMC3957944
- DOI: 10.1128/JVI.03478-13
Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide
Abstract
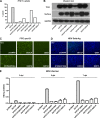
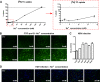
The liver bile acids transporter sodium taurocholate cotransporting polypeptide (NTCP) is responsible for the majority of sodium-dependent bile salts uptake by hepatocytes. NTCP also functions as a cellular receptor for viral entry of hepatitis B virus (HBV) and hepatitis D virus (HDV) through a specific interaction between NTCP and the pre-S1 domain of HBV large envelope protein. However, it remains unknown if these two functions of NTCP are independent or if they interfere with each other. Here we show that binding of the pre-S1 domain to human NTCP blocks taurocholate uptake by the receptor; conversely, some bile acid substrates of NTCP inhibit HBV and HDV entry. Mutations of NTCP residues critical for bile salts binding severely impair viral infection by HDV and HBV; to a lesser extent, the residues important for sodium binding also inhibit viral infection. The mutation S267F, corresponding to a single nucleotide polymorphism (SNP) found in about 9% of the East Asian population, renders NTCP without either taurocholate transporting activity or the ability to support HBV or HDV infection in cell culture. These results demonstrate that molecular determinants critical for HBV and HDV entry overlap with that for bile salts uptake by NTCP, indicating that viral infection may interfere with the normal function of NTCP, and bile acids and their derivatives hold the potential for further development into antiviral drugs.
Importance: Human hepatitis B virus (HBV) and its satellite virus, hepatitis D virus (HDV), are important human pathogens. Available therapeutics against HBV are limited, and there is no drug that is clinically available for HDV infection. A liver bile acids transporter (sodium taurocholate cotransporting polypeptide [NTCP]) critical for maintaining homeostasis of bile acids serves as a functional receptor for HBV and HDV. We report here that the NTCP-binding lipopeptide that originates from the first 47 amino acids of the pre-S1 domain of the HBV L protein blocks taurocholate transport. Some bile salts dose dependently inhibit HBV and HDV infection mediated by NTCP; molecular determinants of NTCP critical for HBV and HDV entry overlap with that for bile acids transport. This work advances our understanding of NTCP-mediated HBV and HDV infection in relation to NTCP's physiological function. Our results also suggest that bile acids or their derivatives hold potential for development into novel drugs against HBV and HDV infection.
Figures





References
-
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 1:e00049. 10.7554/eLife.00049 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

