Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse
- PMID: 24390541
- PMCID: PMC3903209
- DOI: 10.1073/pnas.1313501111
Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse
Abstract
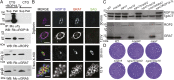
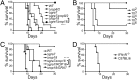
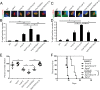
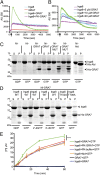
The intracellular parasite Toxoplasma gondii enjoys a wide host range and is adept at surviving in both naive and activated macrophages. Previous studies have emphasized the importance of the active serine-threonine protein kinase rhoptry protein 18 (ROP18), which targets immunity-related GTPases (IRGs), in mediating macrophage survival and acute virulence of T. gondii in mice. Here, we demonstrate that ROP18 exists in a complex with the pseudokinases rhoptry proteins 8 and 2 (ROP8/2) and dense granule protein 7 (GRA7). Individual deletion mutant gra7 or rop18 was partially attenuated for virulence in mice, whereas the combined gra7rop18 mutant was avirulent, suggesting these proteins act together in the same pathway. The virulence defect of the double mutant was mirrored by increased recruitment of IRGs and clearance of the parasite in IFN-γ-activated macrophages in vitro. GRA7 was shown to recognize a conserved feature of IRGs, binding directly to the active dimer of immunity-related GTPase a6 in a GTP-dependent manner. Binding of GRA7 to immunity-related GTPase a6 led to enhanced polymerization, rapid turnover, and eventual disassembly. Collectively, these studies suggest that ROP18 and GRA7 act in a complex to target IRGs by distinct mechanisms that are synergistic.
Keywords: cooperative polymerization; innate immunity; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Dubey JP. Toxoplasmosis of Animals and Humans. Boca Raton, FL: CRC; 2010.
-
- Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. - PubMed
-
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73(2):114–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

