Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis
- PMID: 24390753
- PMCID: PMC4167827
- DOI: 10.1002/hep.26993
Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis
Abstract
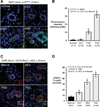
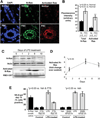
Primary sclerosing cholangitis (PSC) is an incurable cholangiopathy of unknown etiopathogenesis. Here we tested the hypothesis that cholangiocyte senescence is a pathophysiologically important phenotype in PSC. We assessed markers of cellular senescence and senescence-associated secretory phenotype (SASP) in livers of patients with PSC, primary biliary cirrhosis, hepatitis C, and in normals by fluorescent in situ hybridization (FISH) and immunofluorescence microscopy (IFM). We tested whether endogenous and exogenous biliary constituents affect senescence and SASP in cultured human cholangiocytes. We determined in coculture whether senescent cholangiocytes induce senescence in bystander cholangiocytes. Finally, we explored signaling mechanisms involved in cholangiocyte senescence and SASP. In vivo, PSC cholangiocytes expressed significantly more senescence-associated p16(INK4a) and γH2A.x compared to the other three conditions; expression of profibroinflammatory SASP components (i.e., IL-6, IL-8, CCL2, PAI-1) was also highest in PSC cholangiocytes. In vitro, several biologically relevant endogenous (e.g., cholestane 3,5,6 oxysterol) and exogenous (e.g., lipopolysaccharide) molecules normally present in bile induced cholangiocyte senescence and SASP. Furthermore, experimentally induced senescent human cholangiocytes caused senescence in bystander cholangiocytes. N-Ras, a known inducer of senescence, was increased in PSC cholangiocytes and in experimentally induced senescent cultured cholangiocytes; inhibition of Ras abrogated experimentally induced senescence and SASP.
Conclusion: Cholangiocyte senescence induced by biliary constituents by way of N-Ras activation is an important pathogenic mechanism in PSC. Pharmacologic inhibition of N-Ras with a resultant reduction in cholangiocyte senescence and SASP is a new therapeutic approach for PSC.
© 2014 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures






References
-
- Tabibian JH, Lindor KD. Primary sclerosing cholangitis: a review and update on therapeutic developments. Expert Rev Gastroenterol Hepatol. 2013;7:103–114. - PubMed
-
- Wiesner RH, Grambsch PM, Dickson ER, Ludwig J, MacCarty RL, Hunter EB, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–436. - PubMed
-
- Alabraba E, Nightingale P, Gunson B, Hubscher S, Olliff S, Mirza D, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
