Protective MCMV immunity by vaccination of the salivary gland via Wharton's duct: replication-deficient recombinant adenovirus expressing individual MCMV genes elicits protection similar to that of MCMV
- PMID: 24391133
- PMCID: PMC3963010
- DOI: 10.1096/fj.13-244178
Protective MCMV immunity by vaccination of the salivary gland via Wharton's duct: replication-deficient recombinant adenovirus expressing individual MCMV genes elicits protection similar to that of MCMV
Abstract
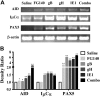
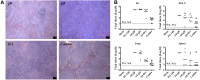
Salivary glands, a major component of the mucosal immune system, confer antigen-specific immunity to mucosally acquired pathogens. We investigated whether a physiological route of inoculation and a subunit vaccine approach elicited MCMV-specific and protective immunity. Mice were inoculated by retrograde perfusion of the submandibular salivary glands via Wharton's duct with tcMCMV or MCMV proteins focused to the salivary gland via replication-deficient adenovirus expressing individual MCMV genes (gB, gH, IE1; controls: saline and replication deficient adenovirus without MCMV inserts). Mice were evaluated for MCMV-specific antibodies, T-cell responses, germinal center formation, and protection against a lethal MCMV challenge. Retrograde perfusion with tcMCMV or adenovirus expressed MCMV proteins induced a 2- to 6-fold increase in systemic and mucosal MCMV-specific antibodies, a 3- to 6-fold increase in GC marker expression, and protection against a lethal systemic challenge, as evidenced by up to 80% increased survival, decreased splenic pathology, and decreased viral titers from 10(6) pfu to undetectable levels. Thus, a focused salivary gland immunization via a physiological route with a protein antigen induced systemic and mucosal protective immune responses. Therefore, salivary gland immunization can serve as an alternative mucosal route for administering vaccines, which is directly applicable for use in humans.
Keywords: inflammation; mucosal immunity; vaccine development.
Figures










Similar articles
-
Salivary gland immunization via Wharton's duct activates differential T-cell responses within the salivary gland immune system.FASEB J. 2019 May;33(5):6011-6022. doi: 10.1096/fj.201801993R. Epub 2019 Feb 28. FASEB J. 2019. PMID: 30817215 Free PMC article.
-
Suppression of murine cytomegalovirus (MCMV) replication with a DNA vaccine encoding MCMV M84 (a homolog of human cytomegalovirus pp65).J Virol. 2000 Apr;74(8):3696-708. doi: 10.1128/jvi.74.8.3696-3708.2000. J Virol. 2000. PMID: 10729145 Free PMC article.
-
Systemic priming-boosting immunization with a trivalent plasmid DNA and inactivated murine cytomegalovirus (MCMV) vaccine provides long-term protection against viral replication following systemic or mucosal MCMV challenge.J Virol. 2005 Jan;79(1):159-75. doi: 10.1128/JVI.79.1.159-175.2005. J Virol. 2005. PMID: 15596812 Free PMC article.
-
The salivary glands as a privileged site of cytomegalovirus immune evasion and persistence.Med Microbiol Immunol. 2008 Jun;197(2):205-13. doi: 10.1007/s00430-008-0077-2. Epub 2008 Feb 8. Med Microbiol Immunol. 2008. PMID: 18259775 Review.
-
CD4 T Cell-Mediated Immune Control of Cytomegalovirus Infection in Murine Salivary Glands.Pathogens. 2021 Nov 23;10(12):1531. doi: 10.3390/pathogens10121531. Pathogens. 2021. PMID: 34959486 Free PMC article. Review.
Cited by
-
Spatial kinetics and immune control of murine cytomegalovirus infection in the salivary glands.PLoS Comput Biol. 2024 Aug 16;20(8):e1011940. doi: 10.1371/journal.pcbi.1011940. eCollection 2024 Aug. PLoS Comput Biol. 2024. PMID: 39150988 Free PMC article.
-
Inhibitory Molecules PD-1, CD73 and CD39 Are Expressed by CD8+ T Cells in a Tissue-Dependent Manner and Can Inhibit T Cell Responses to Stimulation.Front Immunol. 2021 Jul 15;12:704862. doi: 10.3389/fimmu.2021.704862. eCollection 2021. Front Immunol. 2021. PMID: 34335618 Free PMC article.
-
Systemic immune response development in Albino rats after retrograde instillation of COVID-19 vaccine to submandibular salivary gland: An experimental study.J Oral Biol Craniofac Res. 2022 May-Jun;12(3):332-338. doi: 10.1016/j.jobcr.2022.03.013. Epub 2022 Mar 22. J Oral Biol Craniofac Res. 2022. PMID: 35341219 Free PMC article.
-
The salivary gland as a target for enhancing immunization response.Trop Dis Travel Med Vaccines. 2017 Feb 17;3:4. doi: 10.1186/s40794-017-0047-z. eCollection 2017. Trop Dis Travel Med Vaccines. 2017. PMID: 28883974 Free PMC article. Review.
-
The Cellular Localization of Human Cytomegalovirus Glycoprotein Expression Greatly Influences the Frequency and Functional Phenotype of Specific CD4+ T Cell Responses.J Immunol. 2015 Oct 15;195(8):3803-15. doi: 10.4049/jimmunol.1500696. Epub 2015 Sep 11. J Immunol. 2015. PMID: 26363059 Free PMC article. Clinical Trial.
References
-
- Brandtzaeg P. (2007) Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25, 5467–5484 - PubMed
-
- Brandtzaeg P., Baekkevold E. S., Farstad I. N., Jahnsen F. L., Johansen F. E., Nilsen E. M., Yamanaka T. (1999) Regional specialization in the mucosal immune system: what happens in the microcompartments? Immunol. Today 20, 141–151 - PubMed
-
- Macpherson A. J., McCoy K. D., Johansen F. E., Brandtzaeg P. (2008) The immune geography of IgA induction and function. Mucosal. Immunol. 1, 11–22 - PubMed
-
- Takahashi I., Nochi T., Yuki Y., Kiyono H. (2009) New horizon of mucosal immunity and vaccines. Curr. Opin. Immunol. 21, 352–358 - PubMed
-
- Neutra M. R., Kozlowski P. A. (2006) Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6, 148–158 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

