Mitochondrial diaphorases as NAD⁺ donors to segments of the citric acid cycle that support substrate-level phosphorylation yielding ATP during respiratory inhibition
- PMID: 24391134
- PMCID: PMC3963018
- DOI: 10.1096/fj.13-243030
Mitochondrial diaphorases as NAD⁺ donors to segments of the citric acid cycle that support substrate-level phosphorylation yielding ATP during respiratory inhibition
Abstract
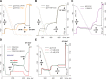
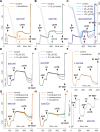
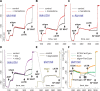
Substrate-level phosphorylation mediated by succinyl-CoA ligase in the mitochondrial matrix produces high-energy phosphates in the absence of oxidative phosphorylation. Furthermore, when the electron transport chain is dysfunctional, provision of succinyl-CoA by the α-ketoglutarate dehydrogenase complex (KGDHC) is crucial for maintaining the function of succinyl-CoA ligase yielding ATP, preventing the adenine nucleotide translocase from reversing. We addressed the source of the NAD(+) supply for KGDHC under anoxic conditions and inhibition of complex I. Using pharmacologic tools and specific substrates and by examining tissues from pigeon liver exhibiting no diaphorase activity, we showed that mitochondrial diaphorases in the mouse liver contribute up to 81% to the NAD(+) pool during respiratory inhibition. Under these conditions, KGDHC's function, essential for the provision of succinyl-CoA to succinyl-CoA ligase, is supported by NAD(+) derived from diaphorases. Through this process, diaphorases contribute to the maintenance of substrate-level phosphorylation during respiratory inhibition, which is manifested in the forward operation of adenine nucleotide translocase. Finally, we show that reoxidation of the reducible substrates for the diaphorases is mediated by complex III of the respiratory chain.
Keywords: DT-diaphorase; adenine nucleotide translocase; reducing equivalent; succinyl-CoA ligase.
Figures




Similar articles
-
Reduction of 2-methoxy-1,4-naphtoquinone by mitochondrially-localized Nqo1 yielding NAD+ supports substrate-level phosphorylation during respiratory inhibition.Biochim Biophys Acta Bioenerg. 2018 Sep;1859(9):909-924. doi: 10.1016/j.bbabio.2018.05.002. Epub 2018 May 7. Biochim Biophys Acta Bioenerg. 2018. PMID: 29746824
-
Methylene blue stimulates substrate-level phosphorylation catalysed by succinyl-CoA ligase in the citric acid cycle.Neuropharmacology. 2017 Sep 1;123:287-298. doi: 10.1016/j.neuropharm.2017.05.009. Epub 2017 May 8. Neuropharmacology. 2017. PMID: 28495375
-
Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage.FASEB J. 2016 Jan;30(1):286-300. doi: 10.1096/fj.15-279398. Epub 2015 Sep 10. FASEB J. 2016. PMID: 26358042
-
Acute sources of mitochondrial NAD+ during respiratory chain dysfunction.Exp Neurol. 2020 May;327:113218. doi: 10.1016/j.expneurol.2020.113218. Epub 2020 Feb 5. Exp Neurol. 2020. PMID: 32035071 Review.
-
Unity and diversity in some bacterial citric acid-cycle enzymes.Adv Microb Physiol. 1981;22:185-244. doi: 10.1016/s0065-2911(08)60328-8. Adv Microb Physiol. 1981. PMID: 7036695 Review. No abstract available.
Cited by
-
Two transgenic mouse models for β-subunit components of succinate-CoA ligase yielding pleiotropic metabolic alterations.Biochem J. 2016 Oct 15;473(20):3463-3485. doi: 10.1042/BCJ20160594. Epub 2016 Aug 5. Biochem J. 2016. PMID: 27496549 Free PMC article.
-
Cancer Stem Cells in Small Cell Lung Cancer Cell Line H446: Higher Dependency on Oxidative Phosphorylation and Mitochondrial Substrate-Level Phosphorylation than Non-Stem Cancer Cells.PLoS One. 2016 May 11;11(5):e0154576. doi: 10.1371/journal.pone.0154576. eCollection 2016. PLoS One. 2016. PMID: 27167619 Free PMC article.
-
GABA synaptopathy promotes the elevation of caspases 3 and 9 as pro-apoptotic markers in Egyptian patients with autism spectrum disorder.Acta Neurol Belg. 2021 Apr;121(2):489-501. doi: 10.1007/s13760-019-01226-z. Epub 2019 Oct 31. Acta Neurol Belg. 2021. PMID: 31673995
-
The Effect of 2-Ketobutyrate on Mitochondrial Substrate-Level Phosphorylation.Neurochem Res. 2019 Oct;44(10):2301-2306. doi: 10.1007/s11064-019-02759-8. Epub 2019 Feb 27. Neurochem Res. 2019. PMID: 30810978 Free PMC article.
-
Localization of SUCLA2 and SUCLG2 subunits of succinyl CoA ligase within the cerebral cortex suggests the absence of matrix substrate-level phosphorylation in glial cells of the human brain.J Bioenerg Biomembr. 2015 Apr;47(1-2):33-41. doi: 10.1007/s10863-014-9586-4. Epub 2014 Nov 5. J Bioenerg Biomembr. 2015. PMID: 25370487 Clinical Trial.
References
-
- Johnson J. D., Mehus J. G., Tews K., Milavetz B. I., Lambeth D. O. (1998) Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J. Biol. Chem. 273, 27580–27586 - PubMed
-
- Chinopoulos C., Gerencser A. A., Mandi M., Mathe K., Torocsik B., Doczi J., Turiak L., Kiss G., Konrad C., Vajda S., Vereczki V., Oh R. J., Adam-Vizi V. (2010) Forward operation of adenine nucleotide translocase during F0F1-ATPase reversal: critical role of matrix substrate-level phosphorylation. FASEB J. 24, 2405–2416 - PMC - PubMed
-
- Chinopoulos C., Adam-Vizi V. (2010) Mitochondria as ATP consumers in cellular pathology. Biochim. Biophys. Acta 1802, 221–227 - PubMed
-
- Chinopoulos C. (2011) Mitochondrial consumption of cytosolic ATP: not so fast. FEBS Lett. 585, 1255–1259 - PubMed
-
- Chinopoulos C. (2011) The “B space” of mitochondrial phosphorylation. J. Neurosci. Res. 89, 1897–1904 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

