High-throughput assay optimization and statistical interpolation of rubella-specific neutralizing antibody titers
- PMID: 24391140
- PMCID: PMC3957678
- DOI: 10.1128/CVI.00681-13
High-throughput assay optimization and statistical interpolation of rubella-specific neutralizing antibody titers
Abstract
Rubella remains a social and economic burden due to the high incidence of congenital rubella syndrome (CRS) in some countries. For this reason, an accurate and efficient high-throughput measure of antibody response to vaccination is an important tool. In order to measure rubella-specific neutralizing antibodies in a large cohort of vaccinated individuals, a high-throughput immunocolorimetric system was developed. Statistical interpolation models were applied to the resulting titers to refine quantitative estimates of neutralizing antibody titers relative to the assayed neutralizing antibody dilutions. This assay, including the statistical methods developed, can be used to assess the neutralizing humoral immune response to rubella virus and may be adaptable for assessing the response to other viral vaccines and infectious agents.
Figures

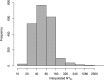
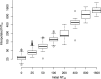
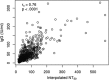
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

