C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America
- PMID: 24391211
- PMCID: PMC3910401
- DOI: 10.4049/jimmunol.1302314
C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America
Abstract
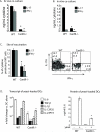
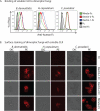
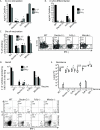
Vaccine immunity to the endemic mycoses of North America requires Th17 cells, but the pattern recognition receptors and signaling pathways that drive these protective responses have not been defined. We show that C-type lectin receptors exert divergent contributions to the development of antifungal Th17 cells and vaccine resistance against Blastomyces dermatitidis, Histoplasma capsulatum, and Coccidioides posadasii. Acquired immunity to B. dermatitidis requires Dectin-2, whereas vaccination against H. capsulatum and C. posadasii infection depends on innate sensing by Dectin-1 and Dectin-2, but not Mincle. Tracking Ag-specific T cells in vivo established that the Card9 signaling pathway acts indispensably and exclusively on differentiation of Th17 cells, while leaving intact their activation, proliferation, survival, and migration. Whereas Card9 signaling is essential, C-type lectin receptors offer distinct and divergent contributions to vaccine immunity against these endemic fungal pathogens. Our work provides new insight into innate immune mechanisms that drive vaccine immunity and Th17 cells.
Figures




Similar articles
-
CARD9-Associated Dectin-1 and Dectin-2 Are Required for Protective Immunity of a Multivalent Vaccine against Coccidioides posadasii Infection.J Immunol. 2020 Jun 15;204(12):3296-3306. doi: 10.4049/jimmunol.1900793. Epub 2020 May 1. J Immunol. 2020. PMID: 32358020 Free PMC article.
-
Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice.J Clin Invest. 2011 Feb;121(2):554-68. doi: 10.1172/JCI43984. Epub 2011 Jan 4. J Clin Invest. 2011. PMID: 21206087 Free PMC article.
-
The C-Type Lectin Receptor MCL Mediates Vaccine-Induced Immunity against Infection with Blastomyces dermatitidis.Infect Immun. 2015 Dec 14;84(3):635-42. doi: 10.1128/IAI.01263-15. Infect Immun. 2015. PMID: 26667836 Free PMC article.
-
Progress in vaccination for histoplasmosis and blastomycosis: coping with cellular immunity.Med Mycol. 2005 Aug;43(5):381-9. doi: 10.1080/13693780500245875. Med Mycol. 2005. PMID: 16178365 Review.
-
Endemic Fungi Presenting as Community-Acquired Pneumonia: A Review.Semin Respir Crit Care Med. 2020 Aug;41(4):522-537. doi: 10.1055/s-0040-1702194. Epub 2020 Jul 6. Semin Respir Crit Care Med. 2020. PMID: 32629490 Review.
Cited by
-
Histoplasma capsulatum, lung infection and immunity.Future Microbiol. 2015;10(6):967-75. doi: 10.2217/fmb.15.25. Future Microbiol. 2015. PMID: 26059620 Free PMC article. Review.
-
Effects of the Dectin-2/TNF-α Pathway on Ventricular Arrhythmia after Acute Myocardial Infarction in Mice.Evid Based Complement Alternat Med. 2022 Aug 11;2022:2521816. doi: 10.1155/2022/2521816. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Dec 13;2023:9819145. doi: 10.1155/2023/9819145. PMID: 35990845 Free PMC article. Retracted.
-
Adaptive immunity to fungi.Cold Spring Harb Perspect Med. 2014 Nov 6;5(3):a019612. doi: 10.1101/cshperspect.a019612. Cold Spring Harb Perspect Med. 2014. PMID: 25377140 Free PMC article. Review.
-
Immune Response to Coccidioidomycosis and the Development of a Vaccine.Microorganisms. 2017 Mar 16;5(1):13. doi: 10.3390/microorganisms5010013. Microorganisms. 2017. PMID: 28300772 Free PMC article. Review.
-
C-type lectin receptors in the control of T helper cell differentiation.Nat Rev Immunol. 2016 Jul;16(7):433-48. doi: 10.1038/nri.2016.55. Epub 2016 Jun 13. Nat Rev Immunol. 2016. PMID: 27291962 Review.
References
-
- Wüthrich M, Filutowicz HI, Warner T, Klein BS. Requisite elements in vaccine immunity to Blastomyces dermatitidis: plasticity uncovers vaccine potential in immune-deficient hosts. J Immunol. 2002;169:6–969-6976. - PubMed
-
- Chai LY, van de Veerdonk F, Marijnissen RJ, Cheng SC, Khoo AL, Hectors M, Lagrou K, Vonk AG, Maertens J, Joosten LA, Kullberg BJ, Netea MG. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130:4–6-54. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

