Speech rhythms and multiplexed oscillatory sensory coding in the human brain
- PMID: 24391472
- PMCID: PMC3876971
- DOI: 10.1371/journal.pbio.1001752
Speech rhythms and multiplexed oscillatory sensory coding in the human brain
Abstract
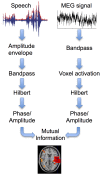
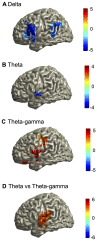
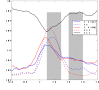
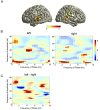
Cortical oscillations are likely candidates for segmentation and coding of continuous speech. Here, we monitored continuous speech processing with magnetoencephalography (MEG) to unravel the principles of speech segmentation and coding. We demonstrate that speech entrains the phase of low-frequency (delta, theta) and the amplitude of high-frequency (gamma) oscillations in the auditory cortex. Phase entrainment is stronger in the right and amplitude entrainment is stronger in the left auditory cortex. Furthermore, edges in the speech envelope phase reset auditory cortex oscillations thereby enhancing their entrainment to speech. This mechanism adapts to the changing physical features of the speech envelope and enables efficient, stimulus-specific speech sampling. Finally, we show that within the auditory cortex, coupling between delta, theta, and gamma oscillations increases following speech edges. Importantly, all couplings (i.e., brain-speech and also within the cortex) attenuate for backward-presented speech, suggesting top-down control. We conclude that segmentation and coding of speech relies on a nested hierarchy of entrained cortical oscillations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
How brain waves help us make sense of speech.PLoS Biol. 2013 Dec;11(12):e1001753. doi: 10.1371/journal.pbio.1001753. Epub 2013 Dec 31. PLoS Biol. 2013. PMID: 24391473 Free PMC article. No abstract available.
References
-
- Siegel M, Donner TH, Engel AK (2012) Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci 13: 121–134. - PubMed
-
- Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6: 285–296. - PubMed
-
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE (2005) An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol 94: 1904–1911. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

