Lysine acetyltransferase GCN5b interacts with AP2 factors and is required for Toxoplasma gondii proliferation
- PMID: 24391497
- PMCID: PMC3879359
- DOI: 10.1371/journal.ppat.1003830
Lysine acetyltransferase GCN5b interacts with AP2 factors and is required for Toxoplasma gondii proliferation
Abstract
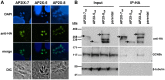
Histone acetylation has been linked to developmental changes in gene expression and is a validated drug target of apicomplexan parasites, but little is known about the roles of individual histone modifying enzymes and how they are recruited to target genes. The protozoan parasite Toxoplasma gondii (phylum Apicomplexa) is unusual among invertebrates in possessing two GCN5-family lysine acetyltransferases (KATs). While GCN5a is required for gene expression in response to alkaline stress, this KAT is dispensable for parasite proliferation in normal culture conditions. In contrast, GCN5b cannot be disrupted, suggesting it is essential for Toxoplasma viability. To further explore the function of GCN5b, we generated clonal parasites expressing an inducible HA-tagged dominant-negative form of GCN5b containing a point mutation that ablates enzymatic activity (E703G). Stabilization of this dominant-negative GCN5b was mediated through ligand-binding to a destabilization domain (dd) fused to the protein. Induced accumulation of the ddHAGCN5b(E703G) protein led to a rapid arrest in parasite replication. Growth arrest was accompanied by a decrease in histone H3 acetylation at specific lysine residues as well as reduced expression of GCN5b target genes in GCN5b(E703G) parasites, which were identified using chromatin immunoprecipitation coupled with microarray hybridization (ChIP-chip). Proteomics studies revealed that GCN5b interacts with AP2-domain proteins, apicomplexan plant-like transcription factors, as well as a "core complex" that includes the co-activator ADA2-A, TFIID subunits, LEO1 polymerase-associated factor (Paf1) subunit, and RRM proteins. The dominant-negative phenotype of ddHAGCN5b(E703G) parasites, considered with the proteomics and ChIP-chip data, indicate that GCN5b plays a central role in transcriptional and chromatin remodeling complexes. We conclude that GCN5b has a non-redundant and indispensable role in regulating gene expression required during the Toxoplasma lytic cycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, et al. (1996) Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84: 843–851. - PubMed
-
- Nagy Z, Tora L (2007) Distinct GCN5/PCAF-containing complexes function as co-activators and are involved in transcription factor and global histone acetylation. Oncogene 26: 5341–5357. - PubMed
-
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, et al. (2007) New nomenclature for chromatin-modifying enzymes. Cell 131: 633–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI092801/AI/NIAID NIH HHS/United States
- R01 AI087625/AI/NIAID NIH HHS/United States
- 1S10RR021056/RR/NCRR NIH HHS/United States
- AI077502/AI/NIAID NIH HHS/United States
- S10 RR019352/RR/NCRR NIH HHS/United States
- T32 GM007491/GM/NIGMS NIH HHS/United States
- S10 RR021056/RR/NCRR NIH HHS/United States
- RC4 AI092801/AI/NIAID NIH HHS/United States
- R56 AI077502/AI/NIAID NIH HHS/United States
- 1S10RR019352/RR/NCRR NIH HHS/United States
- R01 AI077502/AI/NIAID NIH HHS/United States
- AI087625/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

