DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway
- PMID: 24391503
- PMCID: PMC3879357
- DOI: 10.1371/journal.ppat.1003848
DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway
Abstract
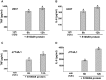
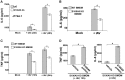
Pathogen-associated molecular patterns (PAMPs) trigger host immune response by activating pattern recognition receptors like toll-like receptors (TLRs). However, the mechanism whereby several pathogens, including viruses, activate TLRs via a non-PAMP mechanism is unclear. Endogenous "inflammatory mediators" called damage-associated molecular patterns (DAMPs) have been implicated in regulating immune response and inflammation. However, the role of DAMPs in inflammation/immunity during virus infection has not been studied. We have identified a DAMP molecule, S100A9 (also known as Calgranulin B or MRP-14), as an endogenous non-PAMP activator of TLR signaling during influenza A virus (IAV) infection. S100A9 was released from undamaged IAV-infected cells and extracellular S100A9 acted as a critical host-derived molecular pattern to regulate inflammatory response outcome and disease during infection by exaggerating pro-inflammatory response, cell-death and virus pathogenesis. Genetic studies showed that the DDX21-TRIF signaling pathway is required for S100A9 gene expression/production during infection. Furthermore, the inflammatory activity of extracellular S100A9 was mediated by activation of the TLR4-MyD88 pathway. Our studies have thus, underscored the role of a DAMP molecule (i.e. extracellular S100A9) in regulating virus-associated inflammation and uncovered a previously unknown function of the DDX21-TRIF-S100A9-TLR4-MyD88 signaling network in regulating inflammation during infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures








References
-
- Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat Immunol 11: 373–384. - PubMed
-
- McGettrick AF, O'Neill LAJ (2010) Localisation and trafficking of toll-like receptors: An important mode of regulation. Curr Opin Immunol 22: 20–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

