Concepts for regulation of axon integrity by enwrapping glia
- PMID: 24391540
- PMCID: PMC3867696
- DOI: 10.3389/fncel.2013.00256
Concepts for regulation of axon integrity by enwrapping glia
Abstract
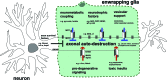
Long axons and their enwrapping glia (EG; Schwann cells (SCs) and oligodendrocytes (OLGs)) form a unique compound structure that serves as conduit for transport of electric and chemical information in the nervous system. The peculiar cytoarchitecture over an enormous length as well as its substantial energetic requirements make this conduit particularly susceptible to detrimental alterations. Degeneration of long axons independent of neuronal cell bodies is observed comparatively early in a range of neurodegenerative conditions as a consequence of abnormalities in SCs and OLGs . This leads to the most relevant disease symptoms and highlights the critical role that these glia have for axon integrity, but the underlying mechanisms remain elusive. The quest to understand why and how axons degenerate is now a crucial frontier in disease-oriented research. This challenge is most likely to lead to significant progress if the inextricable link between axons and their flanking glia in pathological situations is recognized. In this review I compile recent advances in our understanding of the molecular programs governing axon degeneration, and mechanisms of EG's non-cell autonomous impact on axon-integrity. A particular focus is placed on emerging evidence suggesting that EG nurture long axons by virtue of their intimate association, release of trophic substances, and neurometabolic coupling. The correction of defects in these functions has the potential to stabilize axons in a variety of neuronal diseases in the peripheral nervous system and central nervous system (PNS and CNS).
Keywords: Charcot-Marie-Tooth disease; amyotrophic lateral sclerosis; axon; multiple sclerosis; neurodegeneration; oligodendrocyte; schwann cell; wallerian degeneration.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

