Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study
- PMID: 24391803
- PMCID: PMC3877405
- DOI: 10.1371/journal.pone.0083649
Gender differences in clinical outcomes among HIV-positive individuals on antiretroviral therapy in Canada: a multisite cohort study
Abstract
Background: Cohort data examining differences by gender in clinical responses to combination antiretroviral therapy (ART) remain inconsistent and have yet to be explored in a multi-province Canadian setting. This study investigates gender differences by injection drug use (IDU) history in virologic responses to ART and mortality.
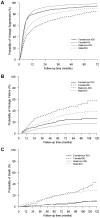
Methods: Data from the Canadian Observational Cohort (CANOC) collaboration, a multisite cohort study of HIV-positive individuals initiating ART after January 1, 2000, were included. This analysis was restricted to participants with a follow-up HIV-RNA plasma viral load measure and known IDU history. Weibull hazard regression evaluated time to virologic suppression (2 consecutive measures <50 copies/mL), rebound (>1000 copies/mL after suppression), and all-cause mortality. Sensitivity analyses explored the impact of presumed ART use in pregnancy on virologic outcomes.
Results: At baseline, women (1120 of 5442 participants) were younger (median 36 vs. 41 years) and more frequently reported IDU history (43.5% vs. 28.8%) (both p<0.001). Irrespective of IDU history, in adjusted multivariable analyses women were significantly less likely to virologically suppress after ART initiation and were at increased risk of viral load rebound. In adjusted time to death analysis, no differences by gender were noted. After adjusting for presumed ART use in pregnancy, observed gender differences in time to virologic suppression for non-IDU, and time to virologic rebound for IDU, became insignificant.
Conclusions: HIV-positive women in CANOC are at heightened risk for poor clinical outcomes. Further understanding of the intersections between gender and other factors augmenting risk is needed to maximize the benefits of ART.
Conflict of interest statement
Figures
References
-
- Public Health Agency of Canada. Summary: Estimates of HIV Prevalence and Incidence in Canada, 2011. Available: www.phac-aspc.gc.ca/aids-sida/publication/survreport/estimat2011-eng.php. Accessed 16 April 2013.
-
- NAIDS. 2012 Report on the Global AIDS Epidemic. Available: www.unaids.org/en/resources/campaigns/20121120_globalreport2012/globalre.... Accessed 16 May 2013.
-
- Collazos J, Asensi V, Carton JA (2007) Sex differences in clinical, immunological and virological parameters of HIV-infected patients treated with HAART. AIDS 21(7): 835–843. - PubMed
-
- Touloumi G, Pantazis N, Babiker AG, Walker SA, Katsarou O, et al. (2004) Differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS 18(12): 1697–1705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical