Comparative characteristics of porous bioceramics for an osteogenic response in vitro and in vivo
- PMID: 24391927
- PMCID: PMC3877265
- DOI: 10.1371/journal.pone.0084272
Comparative characteristics of porous bioceramics for an osteogenic response in vitro and in vivo
Abstract
Porous calcium phosphate ceramics are used in orthopedic and craniofacial applications to treat bone loss, or in dental applications to replace missing teeth. The implantation of these materials, however, does not induce stem cell differentiation, so suitable additional materials such as porous calcium phosphate discs are needed to influence physicochemical responses or structural changes. Rabbit adipose-derived stem cells (ADSC) and mouse osteoblastic cells (MC3T3-E1) were evaluated in vitro by the MTT assay, semi-quantitative RT-PCR, and immunoblotting using cells cultured in medium supplemented with extracts from bioceramics, including calcium metaphosphate (CMP), hydroxyapatite (HA) and collagen-grafted HA (HA-col). In vivo evaluation of the bone forming capacity of these bioceramics in rat models using femur defects and intramuscular implants for 12 weeks was performed. Histological analysis showed that newly formed stromal-rich tissues were observed in all the implanted regions and that the implants showed positive immunoreaction against type I collagen and alkaline phosphatase (ALP). The intramuscular implant region, in particular, showed strong positive immunoreactivity for both type I collagen and ALP, which was further confirmed by mRNA expression and immunoblotting results, indicating that each bioceramic material enhanced osteogenesis stimulation. These results support our hypothesis that smart bioceramics can induce osteoconduction and osteoinduction in vivo, although mature bone formation, including lacunae, osteocytes, and mineralization, was not prominent until 12 weeks after implantation.
Conflict of interest statement
Figures


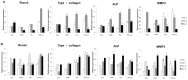




References
-
- Akita S, Tamai N, Myoui A, Nishikawa M, Kaito T, et al. (2004) Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng 10: 789–795 10.1089/1076327041348338.PubMed:15265296 - DOI - PubMed
-
- Ripamonti U (1991) The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am 73: 692–703. PubMed: 1675218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

