Formation of spatially discordant alternans due to fluctuations and diffusion of calcium
- PMID: 24392005
- PMCID: PMC3877395
- DOI: 10.1371/journal.pone.0085365
Formation of spatially discordant alternans due to fluctuations and diffusion of calcium
Abstract
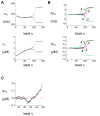
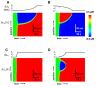
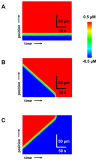
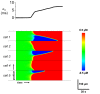
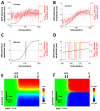
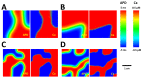
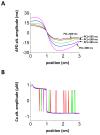
Spatially discordant alternans (SDA) of action potential duration (APD) is a phenomenon where different regions of cardiac tissue exhibit an alternating sequence of APD that are out-of-phase. SDA is arrhythmogenic since it can induce spatial heterogeneity of refractoriness, which can cause wavebreak and reentry. However, the underlying mechanisms for the formation of SDA are not completely understood. In this paper, we present a novel mechanism for the formation of SDA in the case where the cellular instability leading to alternans is caused by intracellular calcium (Ca) cycling, and where Ca transient and APD alternans are electromechanically concordant. In particular, we show that SDA is formed when rapidly paced cardiac tissue develops alternans over many beats due to Ca accumulation in the sarcoplasmic reticulum (SR). The mechanism presented here relies on the observation that Ca cycling fluctuations dictate Ca alternans phase since the amplitude of Ca alternans is small during the early stages of pacing. Thus, different regions of a cardiac myocyte will typically develop Ca alternans which are opposite in phase at the early stages of pacing. These subcellular patterns then gradually coarsen due to interactions with membrane voltage to form steady state SDA of voltage and Ca on the tissue scale. This mechanism for SDA is distinct from well-known mechanisms that rely on conduction velocity restitution, and a Turing-like mechanism known to apply only in the case where APD and Ca alternans are electromechanically discordant. Furthermore, we argue that this mechanism is robust, and is likely to underlie a wide range of experimentally observed patterns of SDA.
Conflict of interest statement
Figures
Similar articles
-
Synchronization of spatially discordant voltage and calcium alternans in cardiac tissue.Phys Rev E. 2022 Aug;106(2-1):024406. doi: 10.1103/PhysRevE.106.024406. Phys Rev E. 2022. PMID: 36109882 Free PMC article.
-
Spatially discordant alternans in cardiac tissue: role of calcium cycling.Circ Res. 2006 Sep 1;99(5):520-7. doi: 10.1161/01.RES.0000240542.03986.e7. Epub 2006 Aug 10. Circ Res. 2006. PMID: 16902177
-
Spatially discordant alternans in cardiomyocyte monolayers.Am J Physiol Heart Circ Physiol. 2008 Mar;294(3):H1417-25. doi: 10.1152/ajpheart.01233.2007. Epub 2008 Jan 25. Am J Physiol Heart Circ Physiol. 2008. PMID: 18223190
-
From pulsus to pulseless: the saga of cardiac alternans.Circ Res. 2006 May 26;98(10):1244-53. doi: 10.1161/01.RES.0000224540.97431.f0. Circ Res. 2006. PMID: 16728670 Review.
-
Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes.J Physiol. 2003 Jan 1;546(Pt 1):19-31. doi: 10.1113/jphysiol.2002.025239. J Physiol. 2003. PMID: 12509476 Free PMC article. Review.
Cited by
-
Cardiac dynamics: Alternans and arrhythmogenesis.J Arrhythm. 2016 Oct;32(5):411-417. doi: 10.1016/j.joa.2016.02.009. Epub 2016 Mar 28. J Arrhythm. 2016. PMID: 27761166 Free PMC article. Review.
-
Synchronization of Triggered Waves in Atrial Tissue.Biophys J. 2018 Sep 18;115(6):1130-1141. doi: 10.1016/j.bpj.2018.08.015. Epub 2018 Aug 18. Biophys J. 2018. PMID: 30195941 Free PMC article.
-
Optical imaging of voltage and calcium in isolated hearts: Linking spatiotemporal heterogeneities and ventricular fibrillation initiation.PLoS One. 2019 May 14;14(5):e0215951. doi: 10.1371/journal.pone.0215951. eCollection 2019. PLoS One. 2019. PMID: 31086382 Free PMC article.
-
Alternans in atria: Mechanisms and clinical relevance.Medicina (Kaunas). 2017;53(3):139-149. doi: 10.1016/j.medici.2017.04.004. Epub 2017 Jun 7. Medicina (Kaunas). 2017. PMID: 28666575 Free PMC article. Review.
-
Dual regulation by subcellular calcium heterogeneity and heart rate variability on cardiac electromechanical dynamics.Chaos. 2020 Sep;30(9):093129. doi: 10.1063/5.0019313. Chaos. 2020. PMID: 33003911 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

