Endocytosis of HERG is clathrin-independent and involves arf6
- PMID: 24392021
- PMCID: PMC3877390
- DOI: 10.1371/journal.pone.0085630
Endocytosis of HERG is clathrin-independent and involves arf6
Abstract
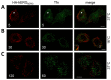
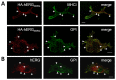
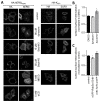
The hERG potassium channel is critical for repolarisation of the cardiac action potential. Reduced expression of hERG at the plasma membrane, whether caused by hereditary mutations or drugs, results in long QT syndrome and increases the risk of ventricular arrhythmias. Thus, it is of fundamental importance to understand how the density of this channel at the plasma membrane is regulated. We used antibodies to an extracellular native or engineered epitope, in conjunction with immunofluorescence and ELISA, to investigate the mechanism of hERG endocytosis in recombinant cells and validated the findings in rat neonatal cardiac myocytes. The data reveal that this channel undergoes rapid internalisation, which is inhibited by neither dynasore, an inhibitor of dynamin, nor a dominant negative construct of Rab5a, into endosomes that are largely devoid of the transferrin receptor. These results support a clathrin-independent mechanism of endocytosis and exclude involvement of dynamin-dependent caveolin and RhoA mechanisms. In agreement, internalised hERG displayed marked overlap with glycosylphosphatidylinositol-anchored GFP, a clathrin-independent cargo. Endocytosis was significantly affected by cholesterol extraction with methyl-β-cyclodextrin and inhibition of Arf6 function with dominant negative Arf6-T27N-eGFP. Taken together, we conclude that hERG undergoes clathrin-independent endocytosis via a mechanism involving Arf6.
Conflict of interest statement
Figures
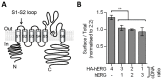
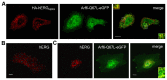

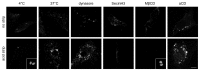
References
-
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, et al. (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113: 365–373 10.1161/CIRCULATIONAHA.105.570200 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

