The alternative role of enterobactin as an oxidative stress protector allows Escherichia coli colony development
- PMID: 24392154
- PMCID: PMC3879343
- DOI: 10.1371/journal.pone.0084734
The alternative role of enterobactin as an oxidative stress protector allows Escherichia coli colony development
Abstract
Numerous bacteria have evolved different iron uptake systems with the ability to make use of their own and heterologous siderophores. However, there is growing evidence attributing alternative roles for siderophores that might explain the potential adaptive advantages of microorganisms having multiple siderophore systems. In this work, we show the requirement of the siderophore enterobactin for Escherichia coli colony development in minimal media. We observed that a strain impaired in enterobactin production (entE mutant) was unable to form colonies on M9 agar medium meanwhile its growth was normal on LB agar medium. Given that, neither iron nor citrate supplementation restored colony growth, the role of enterobactin as an iron uptake-facilitator would not explain its requirement for colony development. The absence of colony development was reverted either by addition of enterobactin, the reducing agent ascorbic acid or by incubating in anaerobic culture conditions with no additives. Then, we associated the enterobactin requirement for colony development with its ability to reduce oxidative stress, which we found to be higher in media where the colony development was impaired (M9) compared with media where the strain was able to form colonies (LB). Since oxyR and soxS mutants (two major stress response regulators) formed colonies in M9 agar medium, we hypothesize that enterobactin could be an important piece in the oxidative stress response repertoire, particularly required in the context of colony formation. In addition, we show that enterobactin has to be hydrolyzed after reaching the cell cytoplasm in order to enable colony development. By favoring iron release, hydrolysis of the enterobactin-iron complex, not only would assure covering iron needs, but would also provide the cell with a molecule with exposed hydroxyl groups (hydrolyzed enterobactin). This molecule would be able to scavenge radicals and therefore reduce oxidative stress.
Conflict of interest statement
Figures



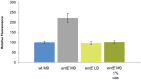


References
-
- Andrews SC, Robinson AK, Rodriguez-Quinones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27: 215–237. - PubMed
-
- Wandersman C, Delepelaire P (2004) Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58: 611–647. - PubMed
-
- Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27: 637–657. - PubMed
-
- Neilands JB (1982) Microbial envelope proteins related to iron. Annu Rev Microbiol 36: 285–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

