3D prostate histology image reconstruction: Quantifying the impact of tissue deformation and histology section location
- PMID: 24392245
- PMCID: PMC3869958
- DOI: 10.4103/2153-3539.120874
3D prostate histology image reconstruction: Quantifying the impact of tissue deformation and histology section location
Abstract
Background: Guidelines for localizing prostate cancer on imaging are ideally informed by registered post-prostatectomy histology. 3D histology reconstruction methods can support this by reintroducing 3D spatial information lost during histology processing. The need to register small, high-grade foci drives a need for high accuracy. Accurate 3D reconstruction method design is impacted by the answers to the following central questions of this work. (1) How does prostate tissue deform during histology processing? (2) What spatial misalignment of the tissue sections is induced by microtome cutting? (3) How does the choice of reconstruction model affect histology reconstruction accuracy?
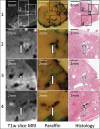
Materials and methods: Histology, paraffin block face and magnetic resonance images were acquired for 18 whole mid-gland tissue slices from six prostates. 7-15 homologous landmarks were identified on each image. Tissue deformation due to histology processing was characterized using the target registration error (TRE) after landmark-based registration under four deformation models (rigid, similarity, affine and thin-plate-spline [TPS]). The misalignment of histology sections from the front faces of tissue slices was quantified using manually identified landmarks. The impact of reconstruction models on the TRE after landmark-based reconstruction was measured under eight reconstruction models comprising one of four deformation models with and without constraining histology images to the tissue slice front faces.
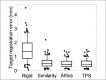
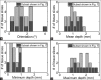
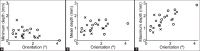
Results: Isotropic scaling improved the mean TRE by 0.8-1.0 mm (all results reported as 95% confidence intervals), while skew or TPS deformation improved the mean TRE by <0.1 mm. The mean misalignment was 1.1-1.9(°) (angle) and 0.9-1.3 mm (depth). Using isotropic scaling, the front face constraint raised the mean TRE by 0.6-0.8 mm.
Conclusions: For sub-millimeter accuracy, 3D reconstruction models should not constrain histology images to the tissue slice front faces and should be flexible enough to model isotropic scaling.
Keywords: 3D histology reconstruction; Correlative histopathology; image registration; prostate cancer imaging; validation.
Figures




References
-
- Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, et al. Advances in magnetic resonance imaging: How they are changing the management of prostate cancer. Eur Urol. 2011;59:962–77. - PubMed
-
- Engelbrecht MR, Barentsz JO, Jager GJ, van der Graaf M, Heerschap A, Sedelaar JP, et al. Prostate cancer staging using imaging. BJU Int. 2000;86(Suppl 1):123–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

