Apoptosis-associated uncoupling of bone formation and resorption in osteomyelitis
- PMID: 24392356
- PMCID: PMC3867676
- DOI: 10.3389/fcimb.2013.00101
Apoptosis-associated uncoupling of bone formation and resorption in osteomyelitis
Abstract
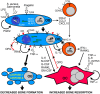
The mechanisms underlying the destruction of bone tissue in osteomyelitis are only now being elucidated. While some of the tissue damage associated with osteomyelitis likely results from the direct actions of bacteria and infiltrating leukocytes, perhaps exacerbated by bacterial manipulation of leukocyte survival pathways, infection-induced bone loss predominantly results from an uncoupling of the activities of osteoblasts and osteoclasts. Bacteria or their products can directly increase osteoclast formation and activity, and the inflammatory milieu at sites of infection can further promote bone resorption. In addition, osteoclast activity is critically regulated by osteoblasts that can respond to bacterial pathogens and foster both inflammation and osteoclastogenesis. Importantly, bone loss during osteomyelitis is also brought about by a decline in new bone deposition due to decreased bone matrix synthesis and by increased rates of osteoblast apoptosis. Extracellular bacterial components may be sufficient to reduce osteoblast viability, but the causative agents of osteomyelitis are also capable of inducing continuous apoptosis of these cells by activating intrinsic and extrinsic cell death pathways to further uncouple bone formation and resorption. Interestingly, bacterial internalization appears to be required for maximal osteoblast apoptosis, and cytosolic inflammasome activation may act in concert with autocrine/paracrine death receptor-ligand signaling to induce cell death. The manipulation of apoptotic pathways in infected bone cells could be an attractive new means to limit inflammatory damage in osteomyelitis. However, the mechanism that is the most important in bacterium-induced bone loss has not yet been identified. Furthermore, it remains to be determined whether the host would be best served by preventing osteoblast cell death or by promoting apoptosis in infected cells.
Keywords: apoptosis; bacterial infection; inflammation; osteoblasts; osteoclasts; osteoimmunology; osteomyelitis.
Figures
Similar articles
-
Staphylococcus aureus biofilms decrease osteoblast viability, inhibits osteogenic differentiation, and increases bone resorption in vitro.BMC Musculoskelet Disord. 2013 Jun 14;14:187. doi: 10.1186/1471-2474-14-187. BMC Musculoskelet Disord. 2013. PMID: 23767824 Free PMC article.
-
Causative agents of osteomyelitis induce death domain-containing TNF-related apoptosis-inducing ligand receptor expression on osteoblasts.Bone. 2011 Apr 1;48(4):857-63. doi: 10.1016/j.bone.2010.11.015. Epub 2010 Dec 3. Bone. 2011. PMID: 21130908
-
Bacterially induced bone destruction: mechanisms and misconceptions.Infect Immun. 1996 Jul;64(7):2371-80. doi: 10.1128/iai.64.7.2371-2380.1996. Infect Immun. 1996. PMID: 8698454 Free PMC article. Review.
-
Cladophora glomerata enriched by biosorption with Mn(II) ions alleviates lipopolysaccharide-induced osteomyelitis-like model in MC3T3-E1, and 4B12 osteoclastogenesis.J Cell Mol Med. 2020 Jul;24(13):7282-7300. doi: 10.1111/jcmm.15294. Epub 2020 Jun 4. J Cell Mol Med. 2020. PMID: 32497406 Free PMC article.
-
Impact of Bacterial Infections on Osteogenesis: Evidence From In Vivo Studies.J Orthop Res. 2019 Oct;37(10):2067-2076. doi: 10.1002/jor.24422. Epub 2019 Aug 11. J Orthop Res. 2019. PMID: 31329305 Free PMC article. Review.
Cited by
-
A Review on Pathophysiology, and Molecular Mechanisms of Bacterial Chondronecrosis and Osteomyelitis in Commercial Broilers.Biomolecules. 2023 Jun 23;13(7):1032. doi: 10.3390/biom13071032. Biomolecules. 2023. PMID: 37509068 Free PMC article. Review.
-
Recent Advances in Research on Antibacterial Metals and Alloys as Implant Materials.Front Cell Infect Microbiol. 2021 Jul 2;11:693939. doi: 10.3389/fcimb.2021.693939. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34277473 Free PMC article. Review.
-
High Molecular Weight Polymer Promotes Bone Health and Prevents Bone Loss Under Salmonella Challenge in Broiler Chickens.Front Physiol. 2018 Apr 13;9:384. doi: 10.3389/fphys.2018.00384. eCollection 2018. Front Physiol. 2018. PMID: 29706903 Free PMC article.
-
The Lyme Disease Pathogen Borrelia burgdorferi Infects Murine Bone and Induces Trabecular Bone Loss.Infect Immun. 2017 Jan 26;85(2):e00781-16. doi: 10.1128/IAI.00781-16. Print 2017 Feb. Infect Immun. 2017. PMID: 27956598 Free PMC article.
-
Efficacy of Silver Nanoparticles-Loaded Bone Cement against an MRSA Induced-Osteomyelitis in a Rat Model.Medicina (Kaunas). 2023 Apr 21;59(4):811. doi: 10.3390/medicina59040811. Medicina (Kaunas). 2023. PMID: 37109771 Free PMC article.
References
-
- Ahmed S., Meghji S., Williams R. J., Henderson B., Brock J. H., Nair S. P. (2001). Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 69, 2872–2877 10.1128/IAI.69.5.2872-2877.2001 - DOI - PMC - PubMed
-
- Alexander E. H., Rivera F. A., Marriott I., Anguita J., Bost K. L., Hudson M. C. (2003). Staphylococcus aureus-induced tumor necrosis factor-related apoptosis-inducing ligand expression mediates apoptosis and caspase-8 activation in infected osteoblasts. BMC Microbiol. 3:5 10.1186/1471-2180-3-5 - DOI - PMC - PubMed
-
- Alexander E. H., Bento J. L., Hughes F. M., Jr., Marriott I., Hudson M. C., Bost K. L. (2001). Staphylococcus aureus and Salmonella enterica serovar Dublin induce tumor necrosis factor-related apoptosis-inducing ligand expression by normal mouse and human osteoblasts, Infect. Immun. 69, 1581–1586 10.1128/IAI.69.3.1581-1586.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

