Plug-and-play fluorophores extend the spectral properties of Spinach
- PMID: 24393009
- PMCID: PMC3929357
- DOI: 10.1021/ja410819x
Plug-and-play fluorophores extend the spectral properties of Spinach
Abstract
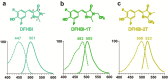
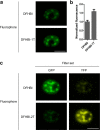
Spinach and Spinach2 are RNA aptamers that can be used for the genetic encoding of fluorescent RNA. Spinach2 binds and activates the fluorescence of (Z)-4-(3,5-difluoro-4-hydroxybenzylidene)-1,2-dimethyl-1H-imidazol-5(4H)-one (DFHBI), allowing the dynamic localizations of Spinach2-tagged RNAs to be imaged in live cells. The spectral properties of Spinach2 are limited by DFHBI, which produces fluorescence that is bluish-green and is not optimized for filters commonly used in fluorescence microscopes. Here we characterize the structural features that are required for fluorophore binding to Spinach2 and describe novel fluorophores that bind and are switched to a fluorescent state by Spinach2. These diverse Spinach2-fluorophore complexes exhibit fluorescence that is more compatible with existing microscopy filter sets and allows Spinach2-tagged constructs to be imaged with either GFP or YFP filter cubes. Thus, these "plug-and-play" fluorophores allow the spectral properties of Spinach2 to be altered on the basis of the specific spectral needs of the experiment.
Figures
References
-
- Tyagi S. Nat. Methods 2009, 6, 331–338. - PubMed
-
- Bertrand E.; Chartrand P.; Schaefer M.; Shenoy S. M.; Singer R. H.; Long R. M. Mol. Cell 1998, 2, 437–445. - PubMed
-
- Babendure J. R.; Adams S. R.; Tsien R. Y. J. Am. Chem. Soc. 2003, 125, 14716–14717. - PubMed
- Constantin T. P.; Silva G. L.; Robertson K. L.; Hamilton T. P.; Fague K.; Waggoner A. S.; Armitage B. A. Org. Lett. 2008, 10, 1561–1564. - PubMed
- Da Costa J. B.; Andreiev A. I.; Dieckmann T. Biochemistry 2013, 52, 6575–6583. - PubMed
- Sando S.; Narita A.; Hayami M.; Aoyama Y. Chem. Commun. (Cambridge, U. K.) 2008, 33, 3858–3860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

