Hypothermia protects human neurons
- PMID: 24393199
- PMCID: PMC4235397
- DOI: 10.1111/ijs.12224
Hypothermia protects human neurons
Abstract
Background and aims: Hypothermia provides neuroprotection after cardiac arrest, hypoxic-ischemic encephalopathy, and in animal models of ischemic stroke. However, as drug development for stroke has been beset by translational failure, we sought additional evidence that hypothermia protects human neurons against ischemic injury.
Methods: Human embryonic stem cells were cultured and differentiated to provide a source of neurons expressing β III tubulin, microtubule-associated protein 2, and the Neuronal Nuclei antigen. Oxygen deprivation, oxygen-glucose deprivation, and H2 O2 -induced oxidative stress were used to induce relevant injury.
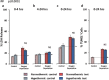
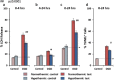
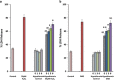
Results: Hypothermia to 33°C protected these human neurons against H2 O2 -induced oxidative stress reducing lactate dehydrogenase release and Terminal deoxynucleotidyl transferase dUTP nick end labeling-staining by 53% (P ≤ 0·0001; 95% confidence interval 34·8-71·04) and 42% (P ≤ 0·0001; 95% confidence interval 27·5-56·6), respectively, after 24 h in culture. Hypothermia provided similar protection against oxygen-glucose deprivation (42%, P ≤ 0·001, 95% confidence interval 18·3-71·3 and 26%, P ≤ 0·001; 95% confidence interval 12·4-52·2, respectively) but provided no protection against oxygen deprivation alone. Protection (21%) persisted against H2 O2 -induced oxidative stress even when hypothermia was initiated six-hours after onset of injury (P ≤ 0·05; 95% confidence interval 0·57-43·1).
Conclusion: We conclude that hypothermia protects stem cell-derived human neurons against insults relevant to stroke over a clinically relevant time frame. Protection against H2 O2 -induced injury and combined oxygen and glucose deprivation but not against oxygen deprivation alone suggests an interaction in which protection benefits from reduction in available glucose under some but not all circumstances.
Keywords: brain; hypothermia; ischemic stroke; neuroprotection; stem cells; treatment.
© 2014 The Authors. International Journal of Stroke published by John Wiley & Sons Ltd on behalf of World Stroke Organization.
Figures


References
-
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. [Meta-Analysis] - PubMed
-
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. - PubMed
-
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. - PubMed
-
- Kollmar R, Gebhardt B, Schwab S. [EuroHYP-1 trial: EU-funded therapy study on the effectiveness of mild therapeutic hypothermia for acute ischemic stroke.] Nervenarzt. 2012;83:1252–1259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

