Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression
- PMID: 24393292
- PMCID: PMC6492994
- DOI: 10.1111/cns.12219
Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression
Abstract
Aims: Adipose tissue releases adipokines that play important roles in metabolic and cardio-cerebro-vascular homeostasis. This study was to discover novel adipokines using caloric restriction model.
Methods: Adipokine candidates were captured by gene array and bioinformatics analysis and verified by preparation of recombinant protein and antibody.
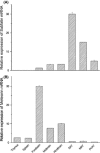
Results: We established a potential secreted protein database containing 208 genes and identified a novel adipokine, Subfatin, that was the highest expressed in subcutaneous fat of both rodents and humans among 15 detected tissues. The secreted mammalian Subfatin was a glycosylated protein. Subfatin was located diffusely throughout the adipose tissue except lipid droplets, with comparable expression between adipocytes and stromal cells, but much lower expression in macrophages than adipocytes. Subfatin was downregulated in white adipose tissue of caloric restriction rats, whereas dramatically upregulated during white adipocyte differentiation as well as in white adipose tissue of diet-induced obese mice. Subfatin was annotated as Meteorin-like (Metrnl) in public databases, a similar transcript of Meteorin (Metrn, also known as glial cell differentiation regulator). Meteorin displayed a brain-specific expression and was scarce in various adipose tissues, in contrast to the tissue expression patterns of Subfatin.
Conclusions: Subfatin is a novel adipokine regulated by adipogenesis and obesity, with tissue distribution different from its homologue Meteorin.
Keywords: Adipokines; Brain; Caloric restriction; Meteorin; Subfatin.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Miao CY. Introduction: Adipokines and cardiovascular disease. Clin Exp Pharmacol Physiol 2011;38:860–863. - PubMed
-
- Flier JS, Maratos‐Flier E. Lasker lauds leptin. Cell 2010;143:9–12. - PubMed
-
- Wang P, Xu TY, Guan YF, et al. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1‐dependent adenosine monophosphate‐activated kinase pathway. Ann Neurol 2011;69:360–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

