Length-dependent processing of telomeres in the absence of telomerase
- PMID: 24393774
- PMCID: PMC3973311
- DOI: 10.1093/nar/gkt1328
Length-dependent processing of telomeres in the absence of telomerase
Abstract
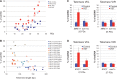
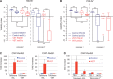
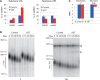
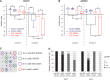
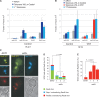
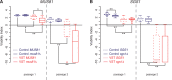
In the absence of telomerase, telomeres progressively shorten with every round of DNA replication, leading to replicative senescence. In telomerase-deficient Saccharomyces cerevisiae, the shortest telomere triggers the onset of senescence by activating the DNA damage checkpoint and recruiting homologous recombination (HR) factors. Yet, the molecular structures that trigger this checkpoint and the mechanisms of repair have remained elusive. By tracking individual telomeres, we show that telomeres are subjected to different pathways depending on their length. We first demonstrate a progressive accumulation of subtelomeric single-stranded DNA (ssDNA) through 5'-3' resection as telomeres shorten. Thus, exposure of subtelomeric ssDNA could be the signal for cell cycle arrest in senescence. Strikingly, early after loss of telomerase, HR counteracts subtelomeric ssDNA accumulation rather than elongates telomeres. We then asked whether replication repair pathways contribute to this mechanism. We uncovered that Rad5, a DNA helicase/Ubiquitin ligase of the error-free branch of the DNA damage tolerance (DDT) pathway, associates with native telomeres and cooperates with HR in senescent cells. We propose that DDT acts in a length-independent manner, whereas an HR-based repair using the sister chromatid as a template buffers precocious 5'-3' resection at the shortest telomeres.
Figures


References
-
- Jain D, Cooper JP. Telomeric strategies: means to an end. Annu. Rev. Genet. 2010;44:243–269. - PubMed
-
- Giraud-Panis MJ, Pisano S, Poulet A, Le Du MH, Gilson E. Structural identity of telomeric complexes. FEBS Lett. 2010;584:3785–3799. - PubMed
-
- Lingner J, Cooper JP, Cech TR. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995;269:1533–1534. - PubMed
-
- Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

