Matrix rigidity regulates spatiotemporal dynamics of Cdc42 activity and vacuole formation kinetics of endothelial colony forming cells
- PMID: 24393843
- PMCID: PMC3971637
- DOI: 10.1016/j.bbrc.2013.12.135
Matrix rigidity regulates spatiotemporal dynamics of Cdc42 activity and vacuole formation kinetics of endothelial colony forming cells
Abstract
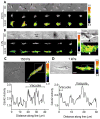
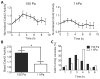
Recent evidence has shown that endothelial colony forming cells (ECFCs) may serve as a cell therapy for improving blood vessel formation in subjects with vascular injury, largely due to their robust vasculogenic potential. The Rho family GTPase Cdc42 is known to play a primary role in this vasculogenesis process, but little is known about how extracellular matrix (ECM) rigidity affects Cdc42 activity during the process. In this study, we addressed two questions: Does matrix rigidity affect Cdc42 activity in ECFC undergoing early vacuole formation? How is the spatiotemporal activation of Cdc42 related to ECFC vacuole formation? A fluorescence resonance energy transfer (FRET)-based Cdc42 biosensor was used to examine the effects of the rigidity of three-dimensional (3D) collagen matrices on spatiotemporal activity of Cdc42 in ECFCs. Collagen matrix stiffness was modulated by varying the collagen concentration and therefore fibril density. The results showed that soft (150 Pa) matrices induced an increased level of Cdc42 activity compared to stiff (1 kPa) matrices. Time-course imaging and colocalization analysis of Cdc42 activity and vacuole formation revealed that Cdc42 activity was colocalized to the periphery of cytoplasmic vacuoles. Moreover, soft matrices generated faster and larger vacuoles than stiff matrices. The matrix-driven vacuole formation was enhanced by a constitutively active Cdc42 mutant, but significantly inhibited by a dominant-negative Cdc42 mutant. Collectively, the results suggest that matrix rigidity is a strong regulator of Cdc42 activity and vacuole formation kinetics, and that enhanced activity of Cdc42 is an important step in early vacuole formation in ECFCs.
Keywords: Endothelial colony forming cells (ECFCs); Fluorescence resonance energy transfer (FRET); Live cell imaging; Matrix stiffness; Mechanotransduction; Rho family GTPases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

