A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity
- PMID: 24394211
- PMCID: PMC4315135
- DOI: 10.1016/j.nano.2013.12.011
A nano-disperse ferritin-core mimetic that efficiently corrects anemia without luminal iron redox activity
Abstract
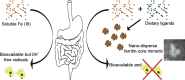
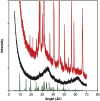
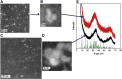
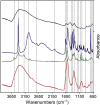
The 2-5 nm Fe(III) oxo-hydroxide core of ferritin is less ordered and readily bioavailable compared to its pure synthetic analogue, ferrihydrite. We report the facile synthesis of tartrate-modified, nano-disperse ferrihydrite of small primary particle size, but with enlarged or strained lattice structure (~2.7Å for the main Bragg peak versus 2.6Å for synthetic ferrihydrite). Analysis indicated that co-precipitation conditions can be achieved for tartrate inclusion into the developing ferrihydrite particles, retarding both growth and crystallization and favoring stabilization of the cross-linked polymeric structure. In murine models, gastrointestinal uptake was independent of luminal Fe(III) reduction to Fe(II) and, yet, absorption was equivalent to that of ferrous sulphate, efficiently correcting the induced anemia. This process may model dietary Fe(III) absorption and potentially provide a side effect-free form of cheap supplemental iron. From the clinical editor: Small size tartrate-modified, nano-disperse ferrihydrite was used for efficient gastrointestinal delivery of soluble Fe(III) without the risk for free radical generation in murine models. This method may provide a potentially side effect-free form iron supplementation.
Keywords: Bioavailability; Ferrihydrite; Iron deficiency anemia; Iron oxide; Oral iron.
Crown Copyright © 2014. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans.Nanomedicine. 2014 Nov;10(8):1877-86. doi: 10.1016/j.nano.2014.06.012. Epub 2014 Jun 28. Nanomedicine. 2014. PMID: 24983890 Free PMC article.
-
A nanoparticulate ferritin-core mimetic is well taken up by HuTu 80 duodenal cells and its absorption in mice is regulated by body iron.J Nutr. 2014 Dec;144(12):1896-902. doi: 10.3945/jn.114.201715. Epub 2014 Oct 23. J Nutr. 2014. PMID: 25342699 Free PMC article.
-
Fe(II) formation after interaction of the amyloid β-peptide with iron-storage protein ferritin.J Biol Phys. 2018 Sep;44(3):237-243. doi: 10.1007/s10867-018-9498-3. Epub 2018 May 9. J Biol Phys. 2018. PMID: 29740739 Free PMC article.
-
Mineralization in ferritin: an efficient means of iron storage.J Struct Biol. 1999 Jun 30;126(3):182-94. doi: 10.1006/jsbi.1999.4118. J Struct Biol. 1999. PMID: 10441528 Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
The Impact of Low-Level Iron Supplements on the Faecal Microbiota of Irritable Bowel Syndrome and Healthy Donors Using In Vitro Batch Cultures.Nutrients. 2020 Dec 14;12(12):3819. doi: 10.3390/nu12123819. Nutrients. 2020. PMID: 33327501 Free PMC article.
-
Advances in Iron Deficiency Anaemia Management: Exploring Novel Drug Delivery Systems and Future Perspectives.Curr Drug Deliv. 2025;22(5):493-509. doi: 10.2174/0115672018300804240426070552. Curr Drug Deliv. 2025. PMID: 39069702 Review.
-
Safety of iron hydroxide adipate tartrate as a novel food pursuant to Regulation (EU) 2015/2283 and as a source of iron in the context of Directive 2002/46/EC.EFSA J. 2021 Dec 10;19(12):e06935. doi: 10.2903/j.efsa.2021.6935. eCollection 2021 Dec. EFSA J. 2021. PMID: 34938369 Free PMC article.
-
Nanoparticulate iron(III) oxo-hydroxide delivers safe iron that is well absorbed and utilised in humans.Nanomedicine. 2014 Nov;10(8):1877-86. doi: 10.1016/j.nano.2014.06.012. Epub 2014 Jun 28. Nanomedicine. 2014. PMID: 24983890 Free PMC article.
-
The Effects of Nanoparticles Containing Iron on Blood and Inflammatory Markers in Comparison to Ferrous Sulfate in Anemic Rats.Int J Prev Med. 2016 Oct 26;7:117. doi: 10.4103/2008-7802.193092. eCollection 2016. Int J Prev Med. 2016. PMID: 27857830 Free PMC article.
References
-
- De Benoist B., McLean E., Egli I., Cogswell M. World Health Organization; Geneva, Switzerland: 2008. Worldwide prevalence of anaemia 1993-2005.
-
- Ezzati M., Lopez A.D., Rodgers A., Vander Hoorn S., Murray C.J. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–1360. - PubMed
-
- WHO . The World Health Report. Reducing risks, promoting healthy life. World Health Organization; Geneva, Switzerland: 2002.
-
- Stoltzfus R.J. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003 Dec;24(4 Suppl):S99–S103. - PubMed
-
- Adamson E.A., Bailey G.R., Richards N., Wilson H. Prevalence of anaemia in an inner city primary school population. Arch Dis Child. 2008;93(5):453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

