Xylitol syrup for the prevention of acute otitis media
- PMID: 24394686
- PMCID: PMC3904279
- DOI: 10.1542/peds.2013-2373
Xylitol syrup for the prevention of acute otitis media
Abstract
Background: Acute otitis media (AOM) is a common childhood illness and the leading indication for antibiotic prescriptions for US children. Xylitol, a naturally occurring sugar alcohol, can reduce AOM when given 5 times per day as a gum or syrup, but a more convenient dosing regimen is needed for widespread adoption.
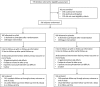
Methods: We designed a pragmatic practice-based randomized controlled trial to determine if viscous xylitol solution at a dose of 5 g 3 times per day could reduce the occurrence of clinically diagnosed AOM among otitis-prone children 6 months through 5 years of age.
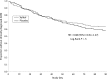
Results: A total of 326 subjects were enrolled, with 160 allocated to xylitol and 166 to placebo. In the primary analysis of time to first clinically diagnosed AOM episode, the hazard ratio for xylitol versus placebo recipients was 0.88 (95% confidence interval [CI] 0.61 to 1.3). In secondary analyses, the incidence of AOM was 0.53 episodes per 90 days in the xylitol group versus 0.59 in the placebo group (difference 0.06; 95% CI -0.25 to 0.13); total antibiotic use was 6.8 days per 90 days in the xylitol group versus 6.4 in the placebo group (difference 0.4; 95% CI -1.8 to 2.7). The lack of effectiveness was not explained by nonadherence to treatment, as the hazard ratio for those taking nearly all assigned xylitol compared with those taking none was 0.93 (95% CI 0.56 to 1.57).
Conclusions: Viscous xylitol solution in a dose of 5 g 3 times per day was ineffective in reducing clinically diagnosed AOM among otitis-prone children.
Keywords: otitis media.
Figures
References
-
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94 - PubMed
-
- Paradise JL, Rockette HE, Colborn DK, et al. . Otitis media in 2253 Pittsburgh-area infants: prevalence and risk factors during the first two years of life. Pediatrics. 1997;99(3):318–333 - PubMed
-
- Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 1997;99(3). Available at: www.pediatrics.org/cgi/content/full/99/3/e1 - PubMed
-
- McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096–3102 - PubMed
-
- Nyquist AC, Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for children with colds, upper respiratory tract infections, and bronchitis. JAMA. 1998;279(11):875–877 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical