A molecular brake controls the magnitude of long-term potentiation
- PMID: 24394804
- PMCID: PMC3895372
- DOI: 10.1038/ncomms4051
A molecular brake controls the magnitude of long-term potentiation
Abstract
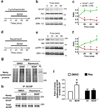
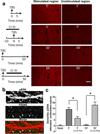
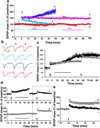
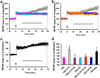
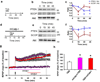
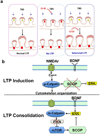
Overexpression of suprachiasmatic nucleus circadian oscillatory protein (SCOP), a negative ERK regulator, blocks long-term memory encoding. Inhibition of calpain-mediated SCOP degradation also prevents the formation of long-term memory, suggesting rapid SCOP breakdown is necessary for memory encoding. However, whether SCOP levels also control the magnitude of long-term synaptic plasticity is unknown. Here we show that following synaptic activity-induced SCOP degradation, SCOP is rapidly replaced via mTOR-mediated protein synthesis. We further show that early SCOP degradation is specifically catalysed by μ-calpain, whereas late SCOP resynthesis is mediated by m-calpain. We propose that μ-calpain promotes long-term potentiation induction by degrading SCOP and activating ERK, whereas m-calpain activation limits the magnitude of potentiation by terminating the ERK response via enhanced SCOP synthesis. This unique braking mechanism could account for the advantages of spaced versus massed training in the formation of long-term memory.
Figures


Similar articles
-
A calpain-2 selective inhibitor enhances learning & memory by prolonging ERK activation.Neuropharmacology. 2016 Jun;105:471-477. doi: 10.1016/j.neuropharm.2016.02.022. Epub 2016 Feb 18. Neuropharmacology. 2016. PMID: 26907807 Free PMC article.
-
Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory.Cell. 2007 Mar 23;128(6):1219-29. doi: 10.1016/j.cell.2006.12.047. Cell. 2007. PMID: 17382888 Free PMC article.
-
SCOPing out proteases in long-term memory.Cell. 2007 Mar 23;128(6):1029-30. doi: 10.1016/j.cell.2007.02.034. Cell. 2007. PMID: 17382874
-
The integrated role of ACh, ERK and mTOR in the mechanisms of hippocampal inhibitory avoidance memory.Neurobiol Learn Mem. 2015 Mar;119:18-33. doi: 10.1016/j.nlm.2014.12.014. Epub 2015 Jan 13. Neurobiol Learn Mem. 2015. PMID: 25595880 Review.
-
Impaired cerebellar plasticity and eye-blink conditioning in calpain-1 knock-out mice.Neurobiol Learn Mem. 2020 Apr;170:106995. doi: 10.1016/j.nlm.2019.02.005. Epub 2019 Feb 5. Neurobiol Learn Mem. 2020. PMID: 30735788 Review.
Cited by
-
Progression in Time of Dentate Gyrus Granule Cell Layer Widening due to Excitotoxicity Occurs along In Vivo LTP Reinstatement and Contextual Fear Memory Recovery.Neural Plast. 2022 Sep 27;2022:7432842. doi: 10.1155/2022/7432842. eCollection 2022. Neural Plast. 2022. PMID: 36213614 Free PMC article.
-
Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning.Brain Res. 2015 Sep 24;1621:73-81. doi: 10.1016/j.brainres.2014.11.033. Epub 2014 Dec 4. Brain Res. 2015. PMID: 25482663 Free PMC article. Review.
-
On the PHLPPside: Emerging roles of PHLPP phosphatases in the heart.Cell Signal. 2021 Oct;86:110097. doi: 10.1016/j.cellsig.2021.110097. Epub 2021 Jul 25. Cell Signal. 2021. PMID: 34320369 Free PMC article. Review.
-
GPR30 Activation Contributes to the Puerarin-Mediated Neuroprotection in MPP+-Induced SH-SY5Y Cell Death.J Mol Neurosci. 2017 Feb;61(2):227-234. doi: 10.1007/s12031-016-0856-y. Epub 2016 Oct 30. J Mol Neurosci. 2017. PMID: 27796870
-
Calpain-1 and Calpain-2: The Yin and Yang of Synaptic Plasticity and Neurodegeneration.Trends Neurosci. 2016 Apr;39(4):235-245. doi: 10.1016/j.tins.2016.01.007. Epub 2016 Feb 10. Trends Neurosci. 2016. PMID: 26874794 Free PMC article. Review.
References
-
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. - PubMed
-
- Winder DG, et al. ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by beta-adrenergic receptors. Neuron. 1999;24:715–726. - PubMed
-
- Shimizu K, Okada M, Nagai K, Fukada Y. Suprachiasmatic nucleus circadian oscillatory protein, a novel binding partner of K-Ras in the membrane rafts, negatively regulates MAPK pathway. J Biol Chem. 2003;278:14920–14925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

